Handbook of Clinical Neurology: Cingulate Cortex; vol. 166, Chapter 1*
2019; Elsevier Publishing
Brent A. Vogt
Cingulum Neurosciences Institute
Handbook of Clinical Neurology: Cingulate Cortex; vol. 166, Chapter 1*
2019; Elsevier Publishing
Brent A. Vogt
Cingulum Neurosciences Institute
While cingulate cortex forms a major medial surface structure surrounding much of the corpus callosum, it has not played a major role in the diagnosis and treatment of neurologic diseases. This chapter seeks to understand why this is the case, provide brief historical insights, and consider cingulate organization. In it I show how to localize the 8 cingulate subregions in any human image of the medial surface. A key theme is that midcingulate cortex (MCC) is not “dorsal anterior cingulate cortex” (“dACC”) as frequently labeled in human imaging studies. There are so many examples of this that only a few can be presented here. *This chapter has been substantially modified from the original.
Broca (1898) early referred to cingulate cortex as the Grand Limbic Lobe thus placing it in the center of what evolved into the limbic concept. Papez (1937) later used large stroke and tumor cases to define what is commonly known as the “Papez circuit” and early emphasized its place in a system rather than a stand-alone structure. This circuit was viewed as a simple feed-forward one from the anterior thalamic nuclei to cingulate cortex to the mammallary bodies and back to the anterior thalamic nuclei leading investigators to view it as one of an internal, reverberating system. While many thought of it as entirely subserving emotion, in fact parts of cingulate cortex including posterior cingulate (PCC) and retrosplenial (RSC) cortices are engaged in spatial orientation and memory that can be in some instances dissociated from emotion.
MacLean (1990), in the summary of his life work, still viewed the thalamocingulate limb of his tripartite brain as critical to emotion but his extensive studies included many sensorimotor responses evoked by electrical stimulation. These latter responses did not become part of his conceptual framework as there were no known mechanisms for them during the period of his active research in the 1960s-1980s. In the end, one of MacLean’s major contributions was to substantially revise the views of Papez by adding the amygdala, orbitofrontal cortex and other subcortical structures to the limbic system. Nevertheless, he still did not have the tools and information whereby sensory information had access to the limbic system or systems by which autonomic/ skeletomotor responses were generated; both of which involved cingulate cortex. This had to await further decades of research with modern neuroscience tools and theoretical frameworks.
The pivotal linkage between autonomic activity and emotion in generating somatic markers (James, 1884; Damasio et al., 1990) suggests that any definition of limbic cortex that depends on emotion requires both autonomic integration as well as long-term memory storage of emotional/ valenced incidents. Only one region in the cingulate gyrus fulfills this definition: anterior cingulate cortex (ACC). A functional designation for the limbic concept involves affect/autonomic activation, emotional responses and memories of valenced objects and events, and mood as it relates to motor activation for emotional motor output. As discussed below, the subgenual ACC (sACC) regulates autonomic output, projects to autonomic brainstem nuclei, and stores long-term, episodic emotional memories and this certainly fulfills the criteria for limbic cortex.
Smith (1907) was the first to recognize a cingulate region that was not identified in Brodmann’s map and we now term midcingulate cortex (MCC; Vogt et al., 2003; Vogt, 2016). Many studies preceded its recognition as an independent entity. For example, electrical stimulation of MCC, that was not yet recognized as such per se, provided the next key generation of research. This stimulation evoked complex and context-dependent gestures such as touching, kneading, rubbing or pressing the fingers or hands together, and lip puckering or sucking; one might say kissing for the latter is context dependent and has emotional valence (Escobedo et al., 1973; Meyer et al., 1973; Talairach et al., 1973). These movements are often adapted to the environment; they can be modified by sensory stimuli, and at times, resisted. None of these complex movements can be evoked by stimulation of adjacent primary and supplementary motor cortices and so are unique to cingulate cortex.
Heiko Braak (1976) observed a motor field in the human posterior cingulate sulcus with pigment (lipofuchian) architecture.  This discovery had a profound and long lasting impact on how the functions of cingulate cortex are viewed particularly when considered with early electrical stimulation studies; i.e., as a premotor/cognitive region that may not always be engaged in emotion or movement. The MCC has two corticospinal projection systems in the cingulate sulcus (Biber et al., 1978; Dum and Strick, 1991, 1993) that are now referred to as the rostral and caudal cingulate premotor areas (CPMAr, CPMAc, respectively). These spinal projection neurons do not extend into ACC, PCC or RSC, and, thus, their presence adds a population of large, layer Vb pyramids that are not characteristic of other parts of cingulate cortex. This architecture is shown in the adjacent figure from Library #3. It shows the caudal cingulate premotor area in area p24dʹ (the prime stands for MCC of which there are two parts; a-anterior and p-posterior). Neuron-specific nuclear binding protein (NeuN) immunoreactivity shows all neurons in the area in the cingulate sulcus, SMI32 is an antibody to intermediate neurofilament proteins that are expressed by the large neurons of Braak in layer Vb and Pigment Architecture (PA) is a preparation kindly provided by Heiko Braak that was used to identify the CPMAc in the cingulate sulcus. Library #3 discusses these findings in a bit more detail. Finally, a summary table of the properties of the two CPMAs is provided at the end of the Library #32 chapter.
This discovery had a profound and long lasting impact on how the functions of cingulate cortex are viewed particularly when considered with early electrical stimulation studies; i.e., as a premotor/cognitive region that may not always be engaged in emotion or movement. The MCC has two corticospinal projection systems in the cingulate sulcus (Biber et al., 1978; Dum and Strick, 1991, 1993) that are now referred to as the rostral and caudal cingulate premotor areas (CPMAr, CPMAc, respectively). These spinal projection neurons do not extend into ACC, PCC or RSC, and, thus, their presence adds a population of large, layer Vb pyramids that are not characteristic of other parts of cingulate cortex. This architecture is shown in the adjacent figure from Library #3. It shows the caudal cingulate premotor area in area p24dʹ (the prime stands for MCC of which there are two parts; a-anterior and p-posterior). Neuron-specific nuclear binding protein (NeuN) immunoreactivity shows all neurons in the area in the cingulate sulcus, SMI32 is an antibody to intermediate neurofilament proteins that are expressed by the large neurons of Braak in layer Vb and Pigment Architecture (PA) is a preparation kindly provided by Heiko Braak that was used to identify the CPMAc in the cingulate sulcus. Library #3 discusses these findings in a bit more detail. Finally, a summary table of the properties of the two CPMAs is provided at the end of the Library #32 chapter.
The topography of the CPMAs and their cytoarchitectural localization was later precisely described in the monkey by Luppino et al. (1991) and Matelli et al. (1991). The CPMAs contain neurons with premotor discharge properties (Shima et al., 1991) that are coded according to the changing reward properties of particular behaviors (Shima and Tanji, 1998; Library #32). Finally, functional imaging studies show altered blood flow in this region during sequences of complex finger apposition movements (Kwan et al., 2000). Thus, the Braak findings opened a stream of important cingulate functional studies that continue to this day.
The field was moved beyond these premotor views to cognitive perspectives by neuropsychology with the concept of “attention-for-action” proposed by Posner et al. (1988) to put the premotor concept in a broader context. This designation is useful because it does not preclude MCC’s role in mismatch detection/conflict resolution (Carter et al., 1998, 2000), selecting among cognitive options that do not require movement (Corbetta et al., 1992; Bench et al., 1992), selecting action verbs to noun lists that may or may not generate movement (Raichle, 2000), anticipation of cognitive processing (Murtha et al., 1996), or working memory (Petit et al., 1998). Involvement of MCC in all of these functions suggests complex intrinsic networks that have yet to be fully understood.
A recent study of cases with aMCC lesions (Fellows and Farah, 2005) raised questions about a strict role for this region in cognitive control. Since Bush et al. (1998) show involvement in enhancing response reaction times and in mediating reward responses (Bush et al., 2002), it is necessary to consider this region in the broader context of response selection toward either reward or away from punishment and this is one of the values of the response selection designation; it can occur toward any outcome and may be determined by feedback parameters that guide behavior (Bush, 2009). Hadland et al. (2003) used monkeys to show that cingulate cortex is involved in selecting responses related to different reward outcomes; although their lesions involved substantial white matter and dorsal supplementary motor cortex. Response selection does not refer to a single cognitive activity as sometimes implied because MCC has many areas and individual layers in each area might contribute to more than one function. Midcingulotomy lesions can disrupt reward-guided response selection (Williams et al., 2004) and the role of MCC in punishment and avoidance is stressed by a substantial literature on acute, peripheral nociceptive stimulation (Vogt et al., 2003, 2005).
A key distinction between ACC and MCC is emotional activity in the former and cognitive/motor activity in the latter. Rolls (2014) defines emotion as states evoked by instrumental reinforcers; rewards or punishers. Emotions set value states (organized in orbitofrontal cortex) that maintain goals for action that are very important features for cingulate cortex. He also proposed a new perspective of limbic system organization (2015) in which he proposes there are at least two limbic systems. One is the anterior limbic system including the orbitofrontal cortex and amygdala that is involved in emotion, reward valuation, and reward-related decision-making (but not memory), with the value representations transmitted to the ACC for action-outcome learning. 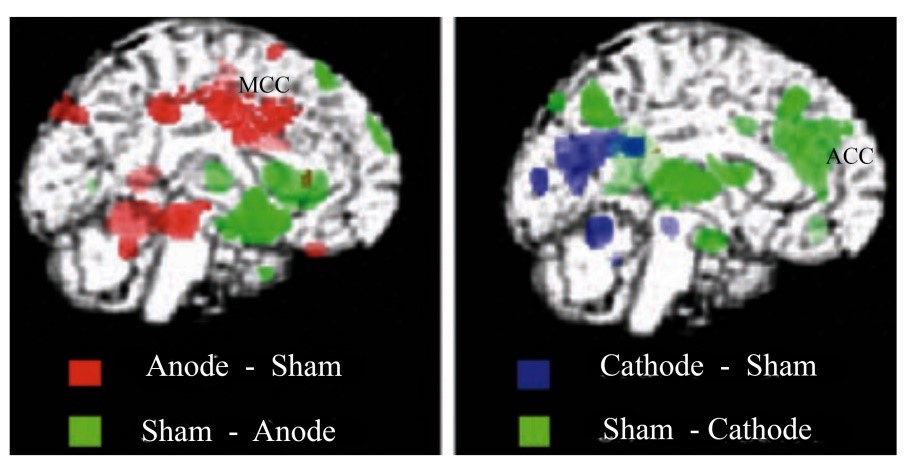 In this ‘emotion limbic system’ a computational principle is that feedforward pattern association networks learn associations from visual, olfactory, somatosensory and auditory stimuli. In the second limbic system the hippocampus and limbic structures with which it is connected including the posterior cingulate cortex (PCC), retrosplenial cortex (RSC) and the fornix-mammillary body-anterior thalamus (i.e., much of the old Papez-MacLean circuit) are involved in episodic, context-related or event memory, but not emotion. This ‘Hippocampal Episodic Memory System’ receives information from neocortical areas about spatial location and objects in space; the hippocampus can rapidly associate this information by the different computational reward value principle of autoassociation in the CA3 region.
In this ‘emotion limbic system’ a computational principle is that feedforward pattern association networks learn associations from visual, olfactory, somatosensory and auditory stimuli. In the second limbic system the hippocampus and limbic structures with which it is connected including the posterior cingulate cortex (PCC), retrosplenial cortex (RSC) and the fornix-mammillary body-anterior thalamus (i.e., much of the old Papez-MacLean circuit) are involved in episodic, context-related or event memory, but not emotion. This ‘Hippocampal Episodic Memory System’ receives information from neocortical areas about spatial location and objects in space; the hippocampus can rapidly associate this information by the different computational reward value principle of autoassociation in the CA3 region.
Finally, it should be known that almost all human imaging studies differentiate ACC and MCC, regardless of a study’s labelling schemes that are usually based on the century-old Brodmann scheme. A particularly provocative demonstration of this comes from the work of Lang et al. (2005). They used transcranial direct current stimulation (tDCS) of primary motor cortex with positron emission tomography as shown in this figure (modified from their Figure 3) at rest and during finger movements and used to map lasting changes in regional synaptic activity following 10 min of tDCS. Subjects received anodal or cathodal stimulation of the left primary motor cortex (MI). When compared to sham stimulation, anodal and cathodal tDCS induced widespread increases and decreases in regional cerebral blood flow (rCBF) in cortical and subcortical areas. These changes in (rCBF) were of the same magnitude as task-related rCBF changes during finger movements. Importantly, anodal stimulation activated MCC, while cathodal stimulation inactivated mainly ACC. These findings clearly show functional differences between these two regions of cingulate cortex.
In conclusion, the midcingulate concept and subregion re-emerged from 1907 throughout the 1990s with a complete cingulate cytoarchitectural study including it but not yet identified as a unique structure/function entity (Vogt et al., 1995) until 2003 (Vogt et al.). MCC provides a cognitive interface with skeletomotor systems via projections to the spinal cord, striatum, supplementary/pre-supplementary motor cortices and other motor systems and is critical to decisions selecting between pain (punishment) or reward outcomes. The response selection process may require movement and/or corollary discharges associated with it, mismatch and/or outcome assessment, assessment of internal requirements and reward consequences, and defining optimal output and reprogramming other motor areas for routine behaviors such as the striatum and cerebellum. The dorsal aMCC (area a32ʹ) may play a pivotal role in reorganizing activity in many motor structures to produce new behavioral outputs that adapt to changing rewards and punishments. The challenge will be to understand how each function is performed within MCC with its many substituent areas.
In the early 1990s, the Brodmann parcellation was still a dominant force particularly as Talairach and Tournoux (1988) used it for their atlas. 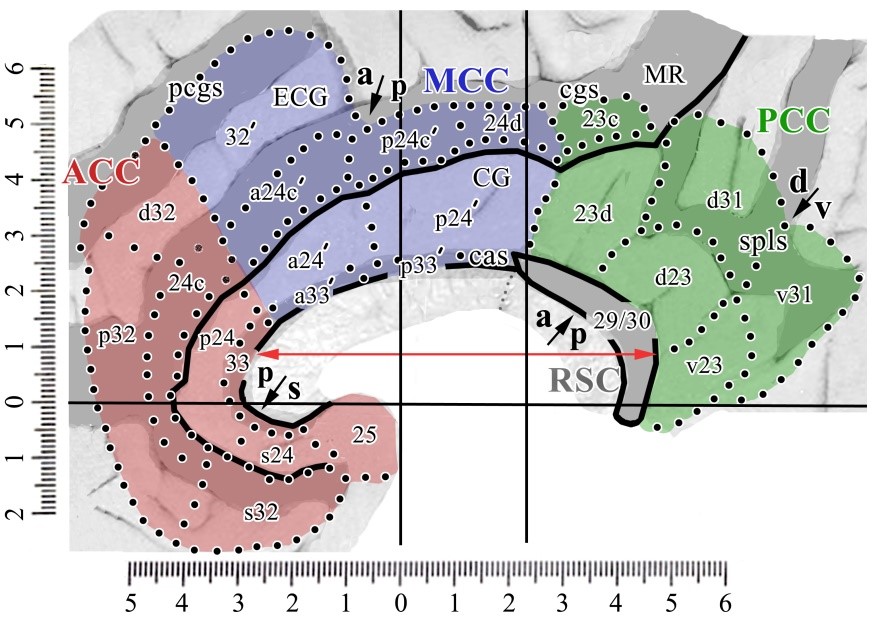 However, almost three decades of structural and functional imaging has provided new perspectives on cingulate organization and use of the label “dACC” has become an incorrect designation for some years, although many investigators continue to use it. The so called “dACC” is not MCC as frequently stated, has no specific and uniform morphological characteristics, and has outlived its usefulness. Indeed, when this label is used, it is unclear where in cingulate cortex the position of structural changes and/or activity is located. The 8-subregion cingulate model is comprised of objectively defined cortical units that are considered further throughout this chapter and does not reflect simple labels but rather models of 8 types of cingulate cortex that have predictive value and their functions will evolve with modern imaging findings. Its predictive value is just being realized as, for example, MCC is one of the main foci of ketamine actions (Sprenger et al., 2006), each of the subregions are qualitatively different cortical types based on differences in neuron structure, distribution of afferent and efferent connections and of course various functions.
However, almost three decades of structural and functional imaging has provided new perspectives on cingulate organization and use of the label “dACC” has become an incorrect designation for some years, although many investigators continue to use it. The so called “dACC” is not MCC as frequently stated, has no specific and uniform morphological characteristics, and has outlived its usefulness. Indeed, when this label is used, it is unclear where in cingulate cortex the position of structural changes and/or activity is located. The 8-subregion cingulate model is comprised of objectively defined cortical units that are considered further throughout this chapter and does not reflect simple labels but rather models of 8 types of cingulate cortex that have predictive value and their functions will evolve with modern imaging findings. Its predictive value is just being realized as, for example, MCC is one of the main foci of ketamine actions (Sprenger et al., 2006), each of the subregions are qualitatively different cortical types based on differences in neuron structure, distribution of afferent and efferent connections and of course various functions.
Over the past two decades we have arrived at the adjacent flat map co-registered to Talairach and Tournoux (1998) coordinates. The four regions (color; ACC, MCC, PCC, RSC), 8 subregions (arrows; subgenual and pregenual ACC; anterior and posterior MCC; dorsal and ventral PCC; anterior/intermediate and posterior RSC) and associated areas are shown.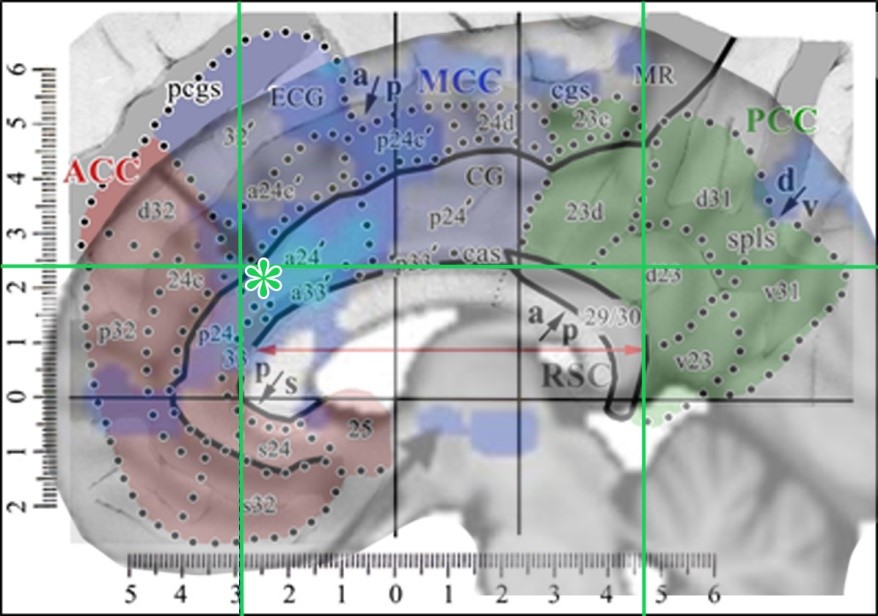 This map is accurate above the corpus callosum where intersection distances are maintained, although sections were extended dorsally to open the cingulate and callosal sulci. However, it is distorted rostral and caudal to the corpus callosum due to the flattening process (Vogt, 2014, 2015). While there are 3 anteroposterior RSC divisions, the anterior and intermediate ones are combined as the anterior division is quite small.
This map is accurate above the corpus callosum where intersection distances are maintained, although sections were extended dorsally to open the cingulate and callosal sulci. However, it is distorted rostral and caudal to the corpus callosum due to the flattening process (Vogt, 2014, 2015). While there are 3 anteroposterior RSC divisions, the anterior and intermediate ones are combined as the anterior division is quite small.
The map has a gray color in the opened sulci and can be used to localize activity on any human image with these steps to generate the composition shown in the figure below: 1. Enter a copy of the above figure into Photoshop. 2. Place the image of interest over the first (in this instance I used the last figure from Hodkinson et al., 2015). 3. Drag two vertical lines to the rostral and caudal tips of the corpus callosum and one horizontal one to the dorsal tip thereof (green lines in figure below). 4. Scale and orient the image to the corpus callosum using these three lines and the red double arrow. 5. Adjust the opacity of the layer with the image of interest so that both images can be viewed simultaneously (in this instance the Hodkinson image was reduced to an opacity of 49%). With this combined image one can relabel any image to the 8-subregion model. 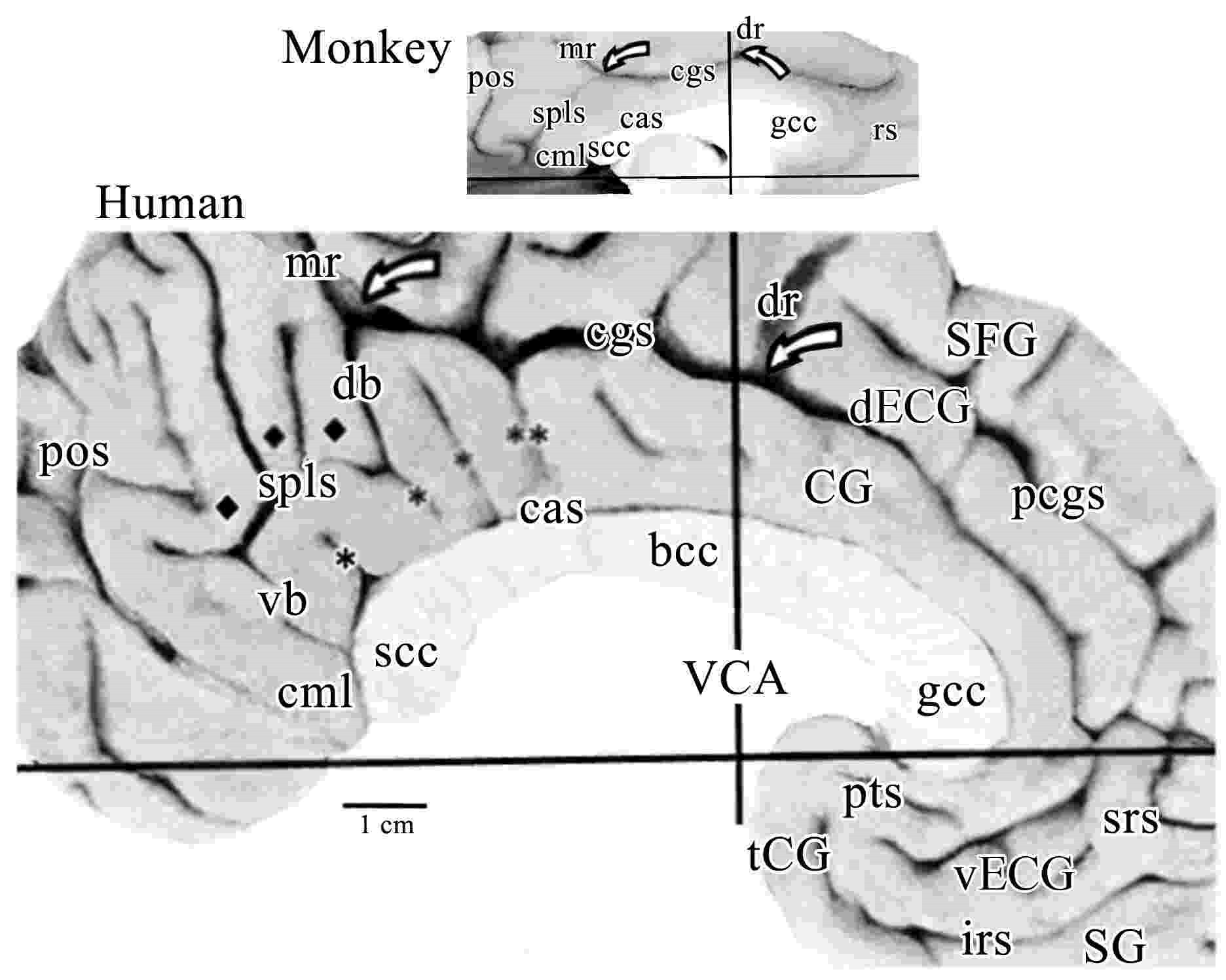 The tip of their arrow in cingulate cortex is marked with a green asterisk and is in aMCC/a24ʹ. All of the observations presented in this chapter support this organization; cytoarchitecture, seeding in PET studies to show associated connections, receptor binding, tDCS, ablation, default mode network, location of Von Economo neurons, cognitive vs emotional processing regions, and projections to subcortical autonomic sites.
The tip of their arrow in cingulate cortex is marked with a green asterisk and is in aMCC/a24ʹ. All of the observations presented in this chapter support this organization; cytoarchitecture, seeding in PET studies to show associated connections, receptor binding, tDCS, ablation, default mode network, location of Von Economo neurons, cognitive vs emotional processing regions, and projections to subcortical autonomic sites.
This figure shows the medial surfaces of the monkey and human brains oriented to the vertical plane at the anterior commissure (VAC or VCA). This human case is among the most common with a double-parallel cingulate and paracingulate sulci. The pairs of open arrows point to the origin of the dorsal rami (dr) and marginal rami (mr). The dorsal (db) and ventral (vb) branches of the splenial sulci (spls) are shown in human. The dorsal splenial lobules are marked with diamonds and vertical branches of the callosal sulcus (cas) in human are marked with asterisks as is a descending branch with double asterisks that marks the approximate border between areas 23 and 24ʹ. While the monkey has almost no branching of the spls, both species have a caudomedial lobule (cml). Monkeys do not have an external cingulate gyrus (ECG; d, dorsal; and v, ventral) or a paracingulate sulcus (pcgs). (Abbreviations include: CG, cingulate gyrus; gcc, bcc, and scc, genu, body and splenium of the corpus callosum, respectively; SFG, superior frontal gyrus; SG, straight gyrus; tCG, terminal cingulate gyrus). The splenial sulci (spls) have a shallow (monkey) or deep (human) secondary sulcus that merges with the cgs at the knee. In human, this branch is named the dorsal branch of the spls to provide a corollary for the ventral branch and to emphasize such continuity. In addition, there is a dorsal ramus (dr) of the cgs just anterior to the VCA in both species. This previously unnamed structure is prominent in human, however, its presence had not been detected in monkey and the dr and mr provide relatively constant markers of the dorsal cingulate gyrus for both species. The human cingulate gyrus has many elaborations not present in the monkey. First and anteriorly, there is the paracingulate sulcus (pcgs) that defines the external cingulate gyrus (ECG) and this gyrus terminates at the dorsal ramus. The caudal one-third of the ECG contains area 32ʹ. Interestingly in the monkey, there is no ECG and no dorsal extension of area 32ʹ. Second, although the monkey has a rostral sulcus (rs), cortex in this region of the human brain is thrown into many folds and these are associated with the inferior and superior rostral sulci, and the paraterminal sulcus (pts) which in this case is broken and continuous with the inferior rostral sulcus. Third, cortex in the human posterior cingulate gyrus is thrown into extensive folds and some of these are termed parasplenial lobules (indicated with diamonds). There are also numerous vertical branches emitted from the spls, cgs, and callosal sulcus (cas) that provide for extensive folding of the PCC in humans. Terminology for the gyrus that surrounds the rostral cingulate gyrus has been a matter of debate over the past few decades. Although Ono et al. (1990) refer to a double pattern of cingulate sulci as common, the dorsal one of the two sulci is quite variable and was not specifically named. Since the paracingulate sulcus defines the dorsal border of this gyrus, it has been referred to as the paracingulate gyrus. This cannot be the case, however, as this cortex is the dorsal ACC that comprises area 32; that is, it is not “para” or adjacent to cingulate cortex. We used the term superior cingulate gyrus (Vogt et al., 1995); however, in its ventral extent below the genu it is not superior to the cingulate gyrus. We resolved these contradictions by referring to it as the external cingulate gyrus and use the paracingulate sulcus to limit the dorsal border of cingulate cortex.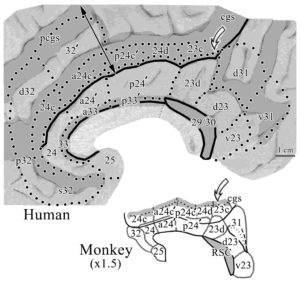
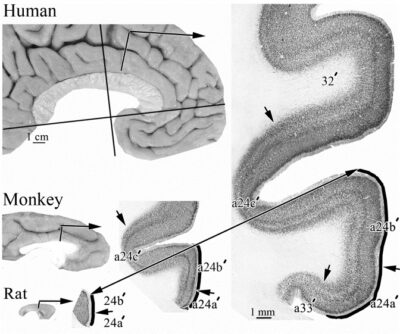
A thorough analysis of the comparative cytoarchitecture is provided in Library #30. Here we make just a few cogent points. This figure shows the areas in both species in flat maps with the double arrow in human indicating the direction of flattening dorsal to the corpus callosum. The following points are notable: 1. Area 32 in monkey does not extend dorsal to the corpus callosum as in human. 2. While the cas does not invaginate in ACC and MCC in monkey as it does in human, we have recently noticed a very limited area 33 in the monkey (not shown). 3. While the CPMAs are in the cingulate sulcus of both species, in the monkey they terminate in the fundus of the cgs (gray area), while in the human these areas extend substantially onto the dorsal bank of the cgs (gray area above the solid line indicating the dorsal edge (apex) of the CG.
This figure shows three species in which sections of NeuN were selected from approximately the same level and magnification for comparison of the aMCC areas. It shows how each species has an expansion of associated areas including the laminar differentiation in primates. The double arrow and solid lines on the surface of the CG enhances these comparative features. A recent article by van Heukelin et al. (2019) makes an explicit effort to provide the comparative features of ACC and MCC in multiple species.
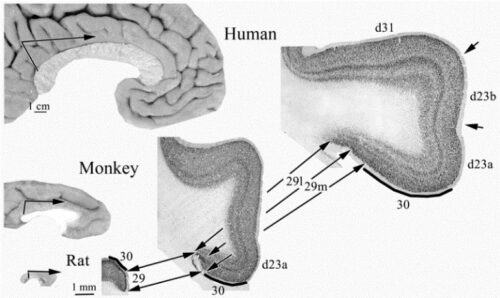
In a similar presentation below, the retrosplenial areas are shown for three species. Note that the rat does not have a PCC; its posterior region is comprised entirely of retrosplenial areas 29 and 30. Further, in primates the retrosplenial areas are almost entirely located in the cas and makes assessing RSC functions quite difficult (below), while in rodents these areas form the entire cortical surface posteriorly without a cas invagination.
The adjacent figure has three plates that show the substantial cytoarchitectural differences between ACC and aMCC (A.), receptor binding differences (B.) and the distribution of the default mode network (C.).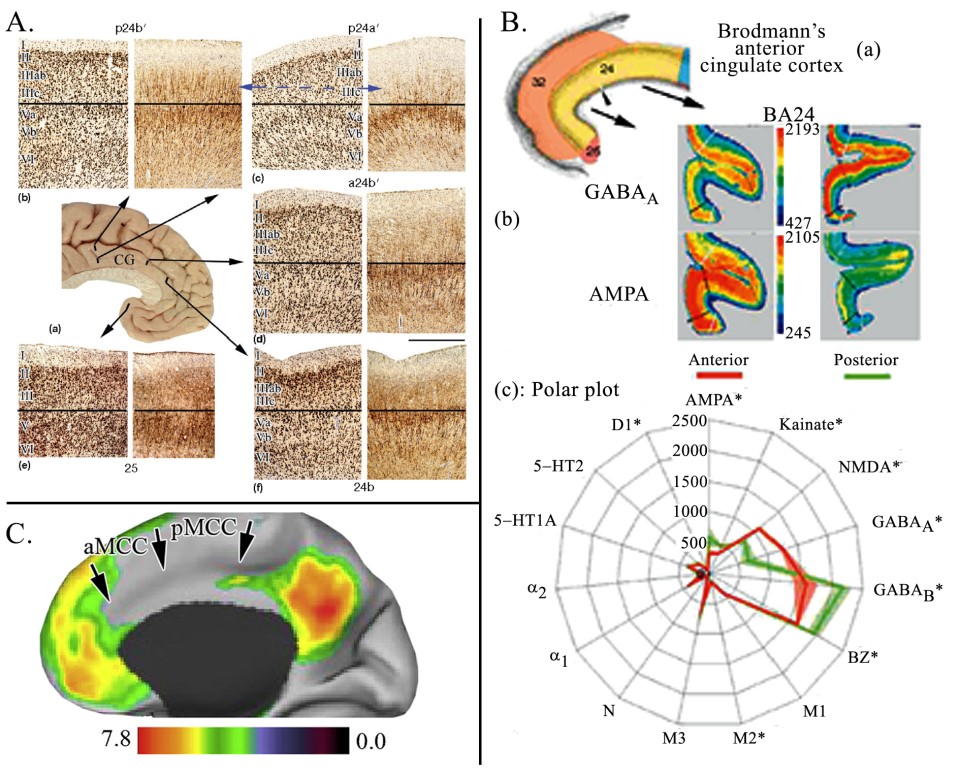 The horizontal black lines in A. mark the boundary between layers III and V in pairs of NeuN and neurofilament protein (NFP; SMI32 antibody) sections. Comparison of each layer in areas progressing from ventral (sACC) to dorsal (p24bʹ) shows a progressive increase in cortical thickness and expansion of particularly the superficial layers. By comparing pACC (f) with aMCC (d) we note the following for aMCC: 1. It is thicker than pACC. 2. Layer II is thinner and less neuron dense. 3. Layers IIIab and IIIc are much broader than in pACC and have more diffuse neurons relating to the expansion of pyramidal basal dendrites. 4. Layer Vb is more apparent and this expansion continues throughout caudal MCC. 5. Neurons in layer VI are larger and the layer is broader. The only layer that appears to have similar features is that of layer Va. It is not apparent how these two cortices can be considered equivalent; i.e., part of the same region. Of particular note is the appearance of NFP in layer IIIc of the latter area. This trend continues into PCC where these neurons become more dense. In B. receptor binding was used to test the hypothesis as to whether or not “dACC” is ACC according to Brodmann’s parcellation (a) (see Library #72). Photographs of GABAA and AMPA receptor binding (b) show robust differences indicating substantial differences in intrinsic circuitry. The statistical analysis for 15 ligands (c) further supports this conclusion. Thus “dACC” is not equivalent to MCC. C. shows that the default mode network clearly does not involve MCC while it does in ACC. This fact has surprisingly been overlooked in the field and supports the notion that “dACC” ≠ ACC.
The horizontal black lines in A. mark the boundary between layers III and V in pairs of NeuN and neurofilament protein (NFP; SMI32 antibody) sections. Comparison of each layer in areas progressing from ventral (sACC) to dorsal (p24bʹ) shows a progressive increase in cortical thickness and expansion of particularly the superficial layers. By comparing pACC (f) with aMCC (d) we note the following for aMCC: 1. It is thicker than pACC. 2. Layer II is thinner and less neuron dense. 3. Layers IIIab and IIIc are much broader than in pACC and have more diffuse neurons relating to the expansion of pyramidal basal dendrites. 4. Layer Vb is more apparent and this expansion continues throughout caudal MCC. 5. Neurons in layer VI are larger and the layer is broader. The only layer that appears to have similar features is that of layer Va. It is not apparent how these two cortices can be considered equivalent; i.e., part of the same region. Of particular note is the appearance of NFP in layer IIIc of the latter area. This trend continues into PCC where these neurons become more dense. In B. receptor binding was used to test the hypothesis as to whether or not “dACC” is ACC according to Brodmann’s parcellation (a) (see Library #72). Photographs of GABAA and AMPA receptor binding (b) show robust differences indicating substantial differences in intrinsic circuitry. The statistical analysis for 15 ligands (c) further supports this conclusion. Thus “dACC” is not equivalent to MCC. C. shows that the default mode network clearly does not involve MCC while it does in ACC. This fact has surprisingly been overlooked in the field and supports the notion that “dACC” ≠ ACC.
Von Economo neurons provide further evidence that ACC/MCC are not equivalent structures as these neurons are primarily located in ACC (Nimchinsky et al., 1995; Library #97). This figure shows they are bipolar in shape (asterisks with arrows) and their apical processes often form aggregates (B., C.). 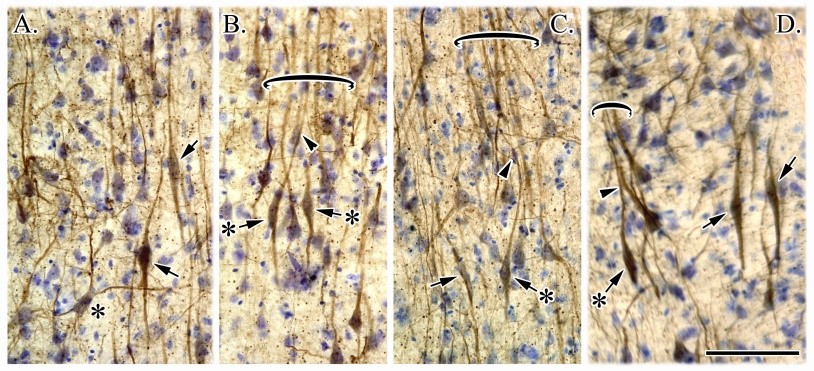 They are large, spindle neurons located mainly in layer Vb. Details regarding the neuronal class to which these cells belong and their precise distribution along both ventrodorsal and anteroposterior axes are now known using computer-assisted mapping and immunocyto-chemical techniques. Spindle neurons are restricted to the subfields of the ACC areas 24 and 25 with a lesser density in MCC. Furthermore, a majority of the spindle cells at any level is located in subarea 24b on the gyral surface. Immunocytochemical analysis shows that NFPs are present in a large percentage of these neurons and that they do not contain calcium-binding proteins. Injections of the carbocyanine dye DiI into the cingulum bundle revealed that these bipolar neurons are projection cells suggesting they may be a type of pyramidal neuron. Finally, spindle cells are consistently affected in Alzheimer’s disease cases, with an overall loss of about 60%. Taken together, these observations indicate that the spindle cells of the human cingulate cortex represent a morphological subpopulation of pyramidal neurons whose restricted distribution may be associated with functions distinct from other projection neurons.
They are large, spindle neurons located mainly in layer Vb. Details regarding the neuronal class to which these cells belong and their precise distribution along both ventrodorsal and anteroposterior axes are now known using computer-assisted mapping and immunocyto-chemical techniques. Spindle neurons are restricted to the subfields of the ACC areas 24 and 25 with a lesser density in MCC. Furthermore, a majority of the spindle cells at any level is located in subarea 24b on the gyral surface. Immunocytochemical analysis shows that NFPs are present in a large percentage of these neurons and that they do not contain calcium-binding proteins. Injections of the carbocyanine dye DiI into the cingulum bundle revealed that these bipolar neurons are projection cells suggesting they may be a type of pyramidal neuron. Finally, spindle cells are consistently affected in Alzheimer’s disease cases, with an overall loss of about 60%. Taken together, these observations indicate that the spindle cells of the human cingulate cortex represent a morphological subpopulation of pyramidal neurons whose restricted distribution may be associated with functions distinct from other projection neurons.
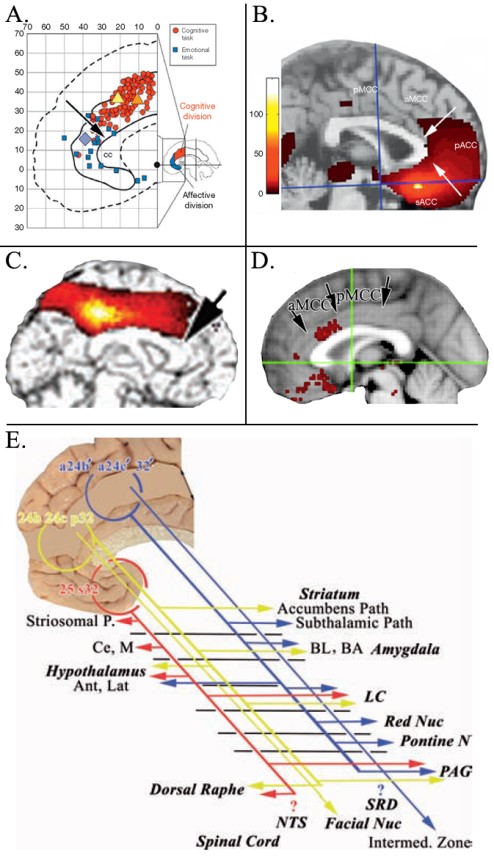 The adjacent figure (A.) provides a summary of Bush (2009) findings for affective (blue) and cognitive cingulate divisions (red) showing that the border between the two aligned almost exactly at the cytoarchitectural border between pACC and aMCC noted above. In B. a seed was placed in the sACC in a PET study (Vogt, 2009) and shows that correlated voxels of interest extend to the same border. In C., a PET study with Steven Laureys (Vogt et al., 2006) and a seed in dPCC shows correlated voxels of interest extending to the same border (black arrow) and not encroaching on ACC. Finally, D. shows “Fear” activity drawn from Neurosynth that is a very restricted patch of activated voxels on the gyral surface of ventral aMCC. We view fear at this level (vs in the amygdala and elsewhere) as a premotor signal that drives avoidance behaviors. Finally, E. shows the autonomic subcortical projections of sACC (red) and pACC (yellow) are more robust and qualitatively unique than that for MCC (blue). The only two similar projections arise from the periaqueductal grey and amygdala. In light of the many findings shown in these figures, it is clear that MCC does not equate to ACC; dorsal, caudal or otherwise.
The adjacent figure (A.) provides a summary of Bush (2009) findings for affective (blue) and cognitive cingulate divisions (red) showing that the border between the two aligned almost exactly at the cytoarchitectural border between pACC and aMCC noted above. In B. a seed was placed in the sACC in a PET study (Vogt, 2009) and shows that correlated voxels of interest extend to the same border. In C., a PET study with Steven Laureys (Vogt et al., 2006) and a seed in dPCC shows correlated voxels of interest extending to the same border (black arrow) and not encroaching on ACC. Finally, D. shows “Fear” activity drawn from Neurosynth that is a very restricted patch of activated voxels on the gyral surface of ventral aMCC. We view fear at this level (vs in the amygdala and elsewhere) as a premotor signal that drives avoidance behaviors. Finally, E. shows the autonomic subcortical projections of sACC (red) and pACC (yellow) are more robust and qualitatively unique than that for MCC (blue). The only two similar projections arise from the periaqueductal grey and amygdala. In light of the many findings shown in these figures, it is clear that MCC does not equate to ACC; dorsal, caudal or otherwise.
There are historical reasons for the limited role that cingulate cortex has played in neurology. It was associated with limbic/emotional systems that in the past were generally viewed to be within the purview of psychiatry and its unique functions were poorly understood. There are, however, other reasons why this is the case. Clinical testing of predictions of its role in motivation and spatial attention has been hindered by the rarity of patients with isolated cingulate lesions (Mesulam et al., 2001). Thus, there has yet to emerge a unique cingulate syndrome and it is often viewed instead as an extension of each adjacent lateral surface neocortical lobe (e.g., medial prefrontal cortex often refers to ACC and dPCC in medial parietal cortex).
There may be as many as 600 neurological disorders evoked by tumors, epilepsy, Parkinson's disease, Alzheimer’s disease, frontotemporal dementia and others. Moreover, there are extensive overlaps between neurological and psychiatric diseases. The latter include mental disorders as a clinically significant behavioral or psychological syndrome or pattern that occurs in an individual and is associated with distress (e.g., a painful symptom) or disability (e.g., impairment in one or more areas of functioning) or with an increased risk of suffering, chronic pain, disability, or an important loss of freedom. Whatever its original etiology, it must currently be considered a manifestation of a behavioral, psychological, or biological dysfunction (Stein et al., 2010). Obviously, numerous neurological diseases are associated with mental/psychiatric disorders and the overlap of these diseases prevents a quantitative assignment of the two. Thus, delineating cingulate damage in one or the other cannot be resolved due to the inextricable nature of forebrain damage and mental impairments. This is further demonstrated with Theory of Mind (ToM) studies.
One way to probe mental function and hence “psychiatric” diseases is with the various paradigms used to analyze Theory of Mind (ToM). These paradigms explore the different mechanisms by which an individual attributes and reasons about the mental states of other individuals in terms of their beliefs and desires particularly in social contexts. The relevant network includes the temporo-parietal junction, cortex in the superior temporal sulcus, dorsal and medial prefrontal cortex and two parts of cingulate cortex (mainly pACC and dPCC along with adjacent area 7 (Dodell-Feder et al., 2011; Kovács et al., 2014). Of course, this network is not unique to ToM processing which overlaps with discourse comprehension (Lin et al., 2018), emotion (Mitchell and Phillips, 2015), and pain (Bruneau et al., 2012). Thus, mental function, as represented by ToM findings, are critical underpinnings for many functions and engage a wide network that includes major parts of cingulate cortex. It is not surprising, therefore, that impairments of cingulate function, such as that in Tourette Syndrome (see below) impair ToM comprehension (Eddy et al., 2016). The conclusion is that neurologic and psychiatric syndromes often go hand in hand.
Attention is without a doubt the overriding view of cingulate function in neurology and this is primarily due to the work of Heilman and colleagues (2003) on contralateral hemineglect. The difficulty of identifying cingulate functions and parceling it in complex diseases is enhanced by the fact that it does not operate as one unified entity and the wide distribution of the anterior cerebral artery (ACA; Stephani et al., 2000; below). This latter study reported that 26% of ACA branches crossed to the other hemisphere, many of which supply the PCC. Thus, infarction of this artery can cause widespread and possibly bilateral insults and it is rare that emboli lodge in a single branch that feed a restricted part (subregion) of cingulate cortex; however, see Paus (2001). While it is certainly true that one has to attend to a task to perform it (i.e., move out of the default mode network), cingulate cortex has a more complex role in brain function than attention and attention itself as a conceptual framework can be refined in cingulate studies (below).
Seizure activity originating in cingulate cortex tends to be silent and difficult to detect. Also, MCC responds during novelty and its function is hard to assess over time. This requires specific neuropsychological tests to assess post-stroke impairments remembering that this damage usually includes substantial white matter including the cingulum bundle with transversely distributed axons, corpus callosum with many axons not limited to cingulate connections and subcortical sites. Finally, cingulate cortex appears to select relevant networks to accomplish particular reinforcement goals and the selection process may be hard to detect independent of other cortical regions and networks. In view of these difficulties, verification of cingulate findings is always required with imaging modalities. Given the skeletomotor functions of MCC, one would expect observable deficits due to damage to it in at least some neurologic diseases, if not as a primary marker of them. There are a few examples of this and here we consider progressive supranuclear palsy (PSP) and Tourette syndrome (TS).
Progressive supranuclear palsy (PSP). Chiu et al. (2012) published an important study differentiating hypoperfusion of MCC in an analysis of PSP and compared it to frontotemporal dementia with tau pathology (FTD). Hypoperfusion associated with PSP was mainly in pMCC but also encroached on aMCC, while that for FTD was mainly in aMCC with some overlap of the two sites. Hypoperfusion in pMCC was correlated with the Stroop color-word and Weigl color-form sorting tests, while that in FTD engaged mainly aMCC and sACC. Thus, the course of PSP is associated with neuron loss and hypoperfusion mainly in pMCC, while that in FTD is in aMCC and sACC (areas 25, s24, s32). Thus, vulnerability of pMCC subregions to neurodegeneration results in cognitive decline in domains usually attributed to aMCC (Bush, 2009). This conundrum has not yet been explained but could be due to neuroplastic reorganization within MCC.
Tourette syndrome (TS). Attention deficit/hyperactivity disorder (ADHD) and obsessive-compulsive disorder (OCD) have a high comorbidity with TS; 60% for the former and 27% for the latter (Freeman et al., 2000). Thus, it is worth going one step further as syndrome overlap could be associated with aspects of MCC impairment. The data, however, are in conflict when viewed from the perspective of the MCC dichotomy. Wang et al. (2011) used independent components analysis of patients with TS during spontaneous tics and healthy controls while simulating tic behaviors. While both groups showed activation in pACC, the activity in aMCC in the TS group was lower than controls and lower activity was associated with more severe tics. This suggests that a failure to control tic behavior or premonitory urges that generate them is due to failure of aMCC function. Bohlhalter et al. (2006) evaluated TS patients with fMRI 2 seconds before and at tic onset without regard to tic type (e.g., eye blinking, grimacing, abdominal tensing, arm stretching, coughing, grunting or barking). They found that pMCC and the supplementary motor area activated before tic onset and, at tic onset, this activity was virtually non-existent. In contrast, significant activation at tic onset was generated in sensorimotor areas. It is striking that the caudal Cingulate Premotor Area (cCPMA) in the pMCC was a focal site of activity. This can be viewed as consistent with the function of pMCC in reflexive motor control versus feedback-mediated decision making of the daMCC and rCPMA. What is not consistent is the reduction in aMCC activity in Wang et al. (2011) and increased pre-tic activity in pMCC (Bohlhalter et al., 2006). There are a number of possible reasons for the mismatch in the two MCC subregions. 1) Both TS populations contained about equal proportions of patients with comorbid OCD and ADHD instead of TS only patients. 2) Each study had small group sizes of 10 and 13 subjects. 3) The tics themselves were variable and focused on those that would not cause motion artifact in the scanner. Selecting a uniform type of tic for analysis could be helpful. 4) As TS evolves with age, a tighter age category may provide more consistent findings. While the available studies are excellent and there are problems finding an adequate number of patients with similar characteristics, the findings leave questions in terms of MCC impairment. This conundrum is an example of how the 8 cingulate subregions provide models for further experimental testing that may lead to a coherent understanding of MCC-impaired function and biomarker(s) of TS and other neurologic diseases.
The arterial supply of cingulate cortex does not follow structure/function divisions and this makes it particularly difficult to identify unique stroke and tumor outcomes. These issues include the wide distribution of the anterior cerebral artery (ACA) including both anterior cingulate cortex (ACC) and MCC, connections between the ACA and internal parietal artery posteriorly and interconnections with vessels in the contralateral hemisphere. The figure here presents two perspectives on cingulate arterial supply. The first (A.) is an excellent study from Stefanie et al. (2000) in which latex was used to fill the arteries. In the top case, the left ACA was filled with yellow latex and supplies the anterior part of the ipsilateral hemisphere. Crossed branches from the right ACA were filled with blue latex that supply the posterior portion of the contra-lateral hemisphere (arrows). In the center case the medial surface of left cerebral hemisphere shows the left ACA (yellow latex) that supplies the anterior part of the ipsilateral hemisphere (open arrow). The crossed branches from the right ACA (large arrows) supply the posterior part of this hemisphere. Note that the paracentral lobule (small arrows) receives the supply from contralateral ACA branches. In the lower case the usual distribution of ACA branches is shown to orbitofrontal (OfA), frontopolar (FpA), anterior (AIFA), medium (MIFA) and posterior (PIFA) internal frontal, paracentral lobule (LPA), superior (SIPA), and inferior (IIPA) internal parietal arteries. Note the presence of branches destined to the contralateral hemisphere (arrows). Also, there are branches destined to the contralateral hemisphere (arrows) (Stefanie et al., 2000). In B. the medial surface at postmortem with intact arachnoid and vessels. Features of PCG are marked as is the vertical/ventral branch of the splenial sulci (vb, arrowhead). Vessels teased from the sulci demonstrate their positions. The pullout shows each of the major vessels supplying the PCG. The bottom plate is a merge of this case with the distribution of the internal parietal artery reported by Marino (1975) and the two apposing arrows indicate the vascular border between the internal parietal and parieto-occipital arteries. The CML, caudomedial lobule, forms the most caudoventral part of the PCG (Vogt and Laureys, 2009). Asterisks locate labeled arteries that were teased from the sulci to demonstrate their positions. The pullout shows each of the major vessels supplying the PCG. The bottom plate is a merge of this case with the distribution of the internal parietal artery reported by Marino (1975) and the two opposing arrows indicate the vascular border between the internal parietal and parieto-occipital arteries (CML, caudomedial lobule forms the most caudoventral part of the PCG). A boundary zone between two blood supplies appears at the vb-splenial sulci (spls) between the inferior parietal branch of the anterior cerebral artery rostrally to dPCC (Perlmutter and Rhoton, 1978) and the parieto-occipital branch of the posterior cerebral artery caudally to the vPCC (Zeal and Rhoton, 1978). Since the internal parietal branch supplies dPCC, the distribution of this artery shown by Marino (1975; branch #8) was co-registered to the postmortem case based on landmarks. These merged images suggest this branch does not supply the vPCC. Although surface assessment provides limited information on specific cortical supplies, such studies confirm that vPCC is supplied by terminal branches of the medial branch of the posterior cerebral artery (Waddington, 1984, pages 48-49; Perlmutter and Rhoton, 1978, Fig. 6; Ring and Waddington, 1968, Figs. 1-3). This segregation of blood supplies verifies the border between dPCC and vPCC and suggests stroke vulnerabilities that are unique to broadly defined subregions.
Crossed branches from the right ACA were filled with blue latex that supply the posterior portion of the contra-lateral hemisphere (arrows). In the center case the medial surface of left cerebral hemisphere shows the left ACA (yellow latex) that supplies the anterior part of the ipsilateral hemisphere (open arrow). The crossed branches from the right ACA (large arrows) supply the posterior part of this hemisphere. Note that the paracentral lobule (small arrows) receives the supply from contralateral ACA branches. In the lower case the usual distribution of ACA branches is shown to orbitofrontal (OfA), frontopolar (FpA), anterior (AIFA), medium (MIFA) and posterior (PIFA) internal frontal, paracentral lobule (LPA), superior (SIPA), and inferior (IIPA) internal parietal arteries. Note the presence of branches destined to the contralateral hemisphere (arrows). Also, there are branches destined to the contralateral hemisphere (arrows) (Stefanie et al., 2000). In B. the medial surface at postmortem with intact arachnoid and vessels. Features of PCG are marked as is the vertical/ventral branch of the splenial sulci (vb, arrowhead). Vessels teased from the sulci demonstrate their positions. The pullout shows each of the major vessels supplying the PCG. The bottom plate is a merge of this case with the distribution of the internal parietal artery reported by Marino (1975) and the two apposing arrows indicate the vascular border between the internal parietal and parieto-occipital arteries. The CML, caudomedial lobule, forms the most caudoventral part of the PCG (Vogt and Laureys, 2009). Asterisks locate labeled arteries that were teased from the sulci to demonstrate their positions. The pullout shows each of the major vessels supplying the PCG. The bottom plate is a merge of this case with the distribution of the internal parietal artery reported by Marino (1975) and the two opposing arrows indicate the vascular border between the internal parietal and parieto-occipital arteries (CML, caudomedial lobule forms the most caudoventral part of the PCG). A boundary zone between two blood supplies appears at the vb-splenial sulci (spls) between the inferior parietal branch of the anterior cerebral artery rostrally to dPCC (Perlmutter and Rhoton, 1978) and the parieto-occipital branch of the posterior cerebral artery caudally to the vPCC (Zeal and Rhoton, 1978). Since the internal parietal branch supplies dPCC, the distribution of this artery shown by Marino (1975; branch #8) was co-registered to the postmortem case based on landmarks. These merged images suggest this branch does not supply the vPCC. Although surface assessment provides limited information on specific cortical supplies, such studies confirm that vPCC is supplied by terminal branches of the medial branch of the posterior cerebral artery (Waddington, 1984, pages 48-49; Perlmutter and Rhoton, 1978, Fig. 6; Ring and Waddington, 1968, Figs. 1-3). This segregation of blood supplies verifies the border between dPCC and vPCC and suggests stroke vulnerabilities that are unique to broadly defined subregions.
Most studies show that ACA infarcts extend into the white matter making it difficult or impossible to identify specific impairments in cingulate subregion function. Bogousslavsky and Regli (1990) reported a few bilateral cases with akinetic mutism, 3 in ACC and two in MCC with this diagnosis. However, without neuropsychological testing, determining structure/function entities are not possible. Paus (2001) summarized a few cases of restricted cingulate damage; one in pACC and two in MCC. Thus, there are a few cases of localized damage in spite of the complex arterial supply.
Finally, “focal” lesions that involve RSC impair memory of spatial-positional relationships and are associated with topokinetic (movement in space) disorientation (Rudge and Warrington, 1991; Takahashi et al., 1997). It should be realized that these strokes encompass both RSC and dPCC as well as the underlying white matter which is a choke point for many axons of passage between cingulate cortex and the medial temporal lobe. This figure shows case 2 in the latter study (white in this figure extending into area 23). This damage is better termed a perisplenial stroke so as to not implicate it with RSC cytoarchitectural areas. There are no strokes limited to RSC and claims to the contrary are based on a misinterpretation of the extent of stroke and RSC cytoarchitecture. Finally, tasks that require subjects to employ memory in relation to previously learned routes in an environment activate RSC and adjacent dPCC (Ghaem et al., 1997; Mellet et al., 2000) and support a role of RSC in the perisplenial complex in body in space orientation functions.
It should be realized that these strokes encompass both RSC and dPCC as well as the underlying white matter which is a choke point for many axons of passage between cingulate cortex and the medial temporal lobe. This figure shows case 2 in the latter study (white in this figure extending into area 23). This damage is better termed a perisplenial stroke so as to not implicate it with RSC cytoarchitectural areas. There are no strokes limited to RSC and claims to the contrary are based on a misinterpretation of the extent of stroke and RSC cytoarchitecture. Finally, tasks that require subjects to employ memory in relation to previously learned routes in an environment activate RSC and adjacent dPCC (Ghaem et al., 1997; Mellet et al., 2000) and support a role of RSC in the perisplenial complex in body in space orientation functions.
Before we address the details of cingulate function and disease, it must be asked on a more global scale, what is the essential function(s) of cingulate cortex? I was asked this question by an investigator at a conference in the 2000s and was surprised at my lack of a crisp and concise answer. As the answer may guide the next generation of cingulate research, it is not a trivial one. Cingulate cortex selects networks to accomplish specific tasks be they achieving a reward or avoiding a punisher. This view places current connection imaging strategies to define cingulate circuits into particular prominence. This conclusion, however, is not as simple as it may appear. Cingulate cortex does not operate as one full unit. It has many functionally independent units that may fulfill the above definition and we still do not fully appreciate how such units interact. Do each of the 8 subregions discussed below have their own extrinsic networks to regulate? How do the component areas contribute to network activation(s)? What information drives each unit and how is it extracted from the rest of the cerebral cortex? These questions and many others remain open.
One of the best studies of human cingulate connections is with MR diffusion tensor imaging (DTI) by Beckmann et al. (2009). They assessed cingulate connectivity with an algorithm to search for regional variations in the probabilistic connectivity profiles of all cingulate voxels with the rest of the brain and 9 subregions emerged. The probabilities of a connection between cingulate cortex and 11 predefined ROIs were also calculated and cingulate voxels with a high probability of connection with the different targets formed separate clusters. An explicit attempt was made to relate the connection clusters to cytoarchitectural entities. However, while these clusters have some correlation with cingulate subregions (Vogt, 2015), there is not an exact overlap. Importantly, there is no reason to expect cingulate inputs, such as those from the amygdala or parietal cortex, to respect cytoarchitectural borders. Further, comparison with monkey findings suggest that about 30% of any one connection has been identified. Thus, human cingulate connectivity studies are getting close to determining the different networks that its subregions regulate.
Lord et al. (2017) have identified PCC as a member of the “rich club” of regions that have extensive connections. The rich club typically includes key regions or hubs from multiple canonical networks, reducing the cost of inter-network communication. The PCC, a hub node embedded within the default mode network, facilitates communication between brain networks and is a key member of the “rich club.” They assessed how metabolic signatures of neuronal integrity and cortical thickness influence the global extent of a functional rich club as measured using the functional rich club coefficient (fRCC). Rich club estimation was performed on functional connectivity of resting state brain BOLD signals. Magnetic resonance spectroscopy was measured in the same session using a point resolved spectroscopy sequence and confirmed convergence of functional rich club with a previously established structural rich club. N-acetyl aspartate (NAA) in PCC is significantly correlated with age, while the rich club coefficient showed no effect of age. In addition, a significant quadratic relationship between fRCC and NAA concentration in PCC was observed. Furthermore, cortical thinning in PCC was correlated with a reduced rich club coefficient after accounting for age and NAA. Thus, the fRCC is related to a marker of neuronal integrity in a key region of the cingulate cortex. Furthermore, cortical thinning in the same area was observed, suggesting that both cortical thinning and neuronal integrity in the hub regions influence functional integration at a whole brain level.
These studies are important starting points to evaluate cingulate connectivity. Future issues must consider the specific information transferred across a particular connection, how the 8 subregions interact and the extent to which one or more of them are engaged in short- or long-term plasticities. Defining connectivities is only the starting point.
This figure provides a broad overview of cingulate cortex functions from Vogt (2014) and raises many of the following issues. 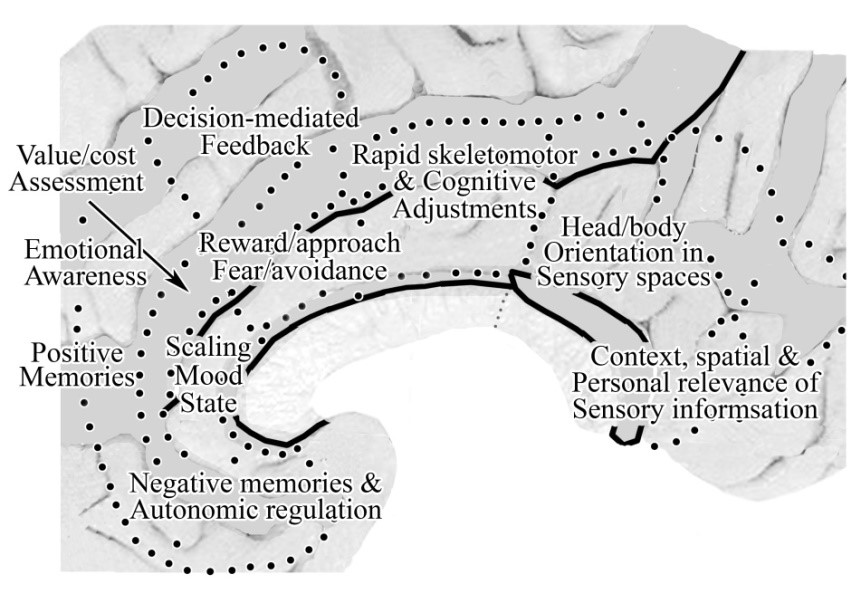 In addition, Caruana et al. (2018) performed a thorough analysis of the functional properties of the entire cingulate cortex by analyzing the effect of high frequency electrical stimulation applied to 1789 cingulate sites in 329 epilepsy patients. The accurate multimodal image-based localiz-ation of stereoelectroencephalography electrodes allowed for a complete map of the specific contributions of each cingulate subregion. Behavioral and subjective responses were subdivided into six main categories: (i) goal-oriented behaviors; (ii) affective; (iii) somatosensory; (iv) vestibular; (v) visual; and (vi) speech impairment in addition to other miscellaneous effects. While their map does not align well with ours, there are a number of interesting confirmations of subregional functions. In this figure A. is their estimation of where cingulate subregions are located with B. showing ours. The main inaccuracies are in ACC and aMCC. The yellow dots in C. are sites that evoked affective responses and they are located mainly in our pACC with a small overlap in aMCC. The yellow dots in B. are sites that evoked goal-oriented behaviors and are mainly in our aMCC. These two findings confirm previous studies as discussed further below and emphasize the value of our borders.
In addition, Caruana et al. (2018) performed a thorough analysis of the functional properties of the entire cingulate cortex by analyzing the effect of high frequency electrical stimulation applied to 1789 cingulate sites in 329 epilepsy patients. The accurate multimodal image-based localiz-ation of stereoelectroencephalography electrodes allowed for a complete map of the specific contributions of each cingulate subregion. Behavioral and subjective responses were subdivided into six main categories: (i) goal-oriented behaviors; (ii) affective; (iii) somatosensory; (iv) vestibular; (v) visual; and (vi) speech impairment in addition to other miscellaneous effects. While their map does not align well with ours, there are a number of interesting confirmations of subregional functions. In this figure A. is their estimation of where cingulate subregions are located with B. showing ours. The main inaccuracies are in ACC and aMCC. The yellow dots in C. are sites that evoked affective responses and they are located mainly in our pACC with a small overlap in aMCC. The yellow dots in B. are sites that evoked goal-oriented behaviors and are mainly in our aMCC. These two findings confirm previous studies as discussed further below and emphasize the value of our borders.  Vestibular sites are shown in red in B. and are located mainly in pMCC confirming its role in rapid orientation to sensory stimuli, while the red dots in C. are sites where visual responses were evoked also confirming previous studies showing a role in viewing personally relevant objects and contexts (Bar and Aminoff, 2003) and also discussed below. The relatively close alignment of affective sites and our pACC and goal-oriented behaviors with aMCC are confirmations of the value of using our subregional map.
Vestibular sites are shown in red in B. and are located mainly in pMCC confirming its role in rapid orientation to sensory stimuli, while the red dots in C. are sites where visual responses were evoked also confirming previous studies showing a role in viewing personally relevant objects and contexts (Bar and Aminoff, 2003) and also discussed below. The relatively close alignment of affective sites and our pACC and goal-oriented behaviors with aMCC are confirmations of the value of using our subregional map.
1. Positive and negative memories of objects and experiences activate areas p32 and s32/s24/25, respectively (Phan et al., 2002; Vogt et al., 2003). Rolls (2014a-Fig. 4.47) and Grabenhorst and Rolls (2011) reinforce and extend this view by demon-strating that aMCC represents negative values produced by a punisher or non-reward that correlate with the subjective state of unpleasantness, while pACC represents positive, reward value that correlates with the subjective state of pleasure. It appears that each of these emotion states are dependent on OFC which plays a major role in cingulate functions by setting value states that undergird action outcomes. Additionally, the sACC has direct projections in autonomic regulatory centers including the lateral hypothalamus, amygdala, periaqueductal grey, and parabrachial nucleus (Vogt and Vogt, 2009). It is likely, therefore, that somatic markers, as conceptualized by Damasio (1996), are of particular relevance to this region where autonomic function is sensed and regulated as noted above. Notice that MCC and PCC do not have most of these projections. Only the MCC has periaqueductal grey and amygdala projections.
2. Emotional awareness is reflected by activations in pACC area 32 and adjacent medial prefrontal cortex. Lane et al. (1997) examined neural activation associated with attending to one's own emotional experience. By having subjects attend to and valence of their experience during assessment of pictures expressing different valences, they examined reflective awareness. Assessment of internal emotional states by this region including part of area 24 is supported by Grabenhorst et al. (2008) and Rolls (2014a) who showed this region is active when making continuous versus binary decisions about the pleasantness or aversiveness of sensory stimuli.
3. Common value scaling is critical for selecting action outcomes. Rolls notes that in order to select a specific goal during decision making, neuronal activity must be scaled in the same value range (Rolls, 2014a). Grabenhorst et al. (2010) showed that fundamentally different primary rewards (e.g., taste in the mouth and warmth on the hand) evoked responses in OFC and pACC that were scaled to the same range. Thus, different rewards (and punishers) are expressed on a similar scale for decision making in pACC area 24 to which many emotional states have access including nociceptive responses (Kulkarni et al., 2005; Vogt, 2005). This function may be a core to motivated behavior and intermediate between the remainder of ACC and aMCC functions.
4. Cost assessment of actions is a critical feature of decision making mediated by pACC area 24c. Hayden et al. (2011) explored this area in monkeys during foraging in a virtual reality task. Single neurons encoded a decision variable signaling the relative value of leaving a depleting resource for a new one. Neurons fired during each sequential decision to stay in a patch and, for each travel time, these responses reached a threshold for patch-leaving. Longer travel times reduced the gain of neural responses for choosing to stay in a patch and increased firing rate threshold mandating patch-leaving. As area 24c may be the rostral end of the rCPMA representing the face and head, it is not surprising that such an area would take the lead in decisions relating to identifying sources of food and water.
1. Feedback-mediated decision making for reward/approach and fear/avoidance selection is a key function of aMCC and much of cingulate information processing likely focusses on the function of this subregion. Bush (2009) discussed the role of dorsal aMCC (areas a24c' and 32') in feedback-mediated decision making. He emphasized that no single “unimodal” theory accounts for the functions of this subregion. Thus, novelty and error detection, anticipation, and target and response selection are all subserved by it and it is a critical hub mediating final decisions. Huster et al. (2011) showed independent components that were significantly correlated with the single-trial electroen-cephalography components for go-, stop- and error-trials and the aMCC maxima in fMRI were greatest on stop- and error-related conditions; hence the notion of feedback-mediation of decisions. One of the keys to the significance of MCC is its activation by a few emotion-generating stimuli (fear and reward) and decisions involving cognitive (non-valenced) processing. Shima and Tanji (1998) reported neurons that encode reward-based decision making including target detection, motor response and reduced rewards with very few neurons responding to constant rewards. This latter finding was later confirmed in humans by Bush et al. (2002) in a study that was designed around findings in monkeys. Finally, the intermingling of nociceptive and reward coding neurons reported by Koyama et al. (2001) emphasizes the important point that this is the final common site for selecting between reward/approach and fear/avoidance behaviors and a mechanism for differentiating these two fundamentally different functions has yet to be proposed. Thus, the cost of actions and their outcomes is determined in areas 24c' and 32'.
2. Rapid cognitive and skeletomotor adjustments are made in pMCC that do not reflect internal emotional assessments of sensory stimuli. The pMCC is activated very early in the nociceptive response (Frot et al., 2008) occurring within 200-300 milliseconds before conscious cognitive processing occurs. The pre-movement responses of neurons in the caudal cingulate premotor area are much shorter than those of the rostral premotor region suggesting there is a system for more rapid orienting responses. Grabenhorst et al. (2008) evaluated graded or yes-no responses to pleasant warm, noxious cold or combinations thereof and pMCC had greater activations on yes vs. no trials suggesting a limited level of cognitive processing. These activations were related to a yes decision, and not to the particular response. Importantly, this subregion did not reflect the pleasantness of the stimuli when ratings were being made, so their activations are related to a ‘Go’ decision, compared with a decision not to Go. Further, the Cingulate Premotor Pain Model (Vogt and Sikes, 2009) proposes that the pMCC is involved in rapid adjustments based on early nociceptive responses that occur within 200-300 milliseconds that are not engaged in pain affect (Bentley et al., 2003; Frot et al., 2008). Also, the features of cCPMA neuron responses indicate earlier premotor activity in pMCC than in aMCC (Shima and Tanji, 1998) reflecting rapid responses on rewarded trials with less intervening cognitive/emotional modulation. Finally, Mohr et al. (2005) showed three parts of cingulate cortex are differentially activated by either externally or self-administered noxious stimuli with pMCC activation during externally generated noxious stimuli; suggesting a limited intervention of internal emotional assessment. There are virtually no reports of emotional activity in MCC; only fear in aMCC (Phan et al., 2002; Vogt et al., 2003).
1. Eye, head and body orientation in sensory spaces are coded by neurons in dPCC. The position of the eye in the orbit was reported by Olson et al. (1996). Richer et al. (1993) electrically stimulated PCC in epileptic patients and evoked complex proprioceptive sensations in the form of bilateral feelings of levitation unaccompanied by movement. It appears that activity in dPCC provides a pre-potent signal that prepares for withdrawal responses should one be required. Another perspective on this function is provided by Mesulam et al. (2001) in which they show that dPCC and adjacent RSC promote the speed of spatial target detection to coordinate spatial attention by modulating expectancy and motivational valence.
2. The vPCC is engaged in assessing the context-spatial- and personal-relevance of sensory information; i.e., sensory information is not captured by vPCC unless it has personal significance. A very important study by Bar and Aminoff (2003) scanned subjects while they observed highly contextualized objects (e.g., a beach chair on the beach vs objects independent of contexts) and suggested that activated cortices mediate spatial and non-spatial contexts. Once the personal relevance of an object or experience has been coded in vPCC, it becomes available to sACC via direct connections for combining value and actions in ACC/MCC (e.g., sitting on the beach chair). See the connection between vPCC and ACC below. Given the interconnection of vPCC and sACC, it should be noted that personal relevance is also coded in pACC area 24. Enzi et al. (2009) showed that responses evoked by reward and the attribution of personal relevance during reward and personal relevance included the pACC. Although we showed basal glucose correlations between vPCC and OFC (Vogt et al., 2006), Rolls’ perspectives on value representations in OFC has required a more explicit connection between OFC and vPCC that participates in the personal relevance proposition. Thus, the interactions among vPCC, pACC and OFC are critical to defining ensuing decision making.
This figure shows graphics from Vogt (2005; A.) and current updates from Neurosynth (Yarkoni et al., 2011; B.-C.). 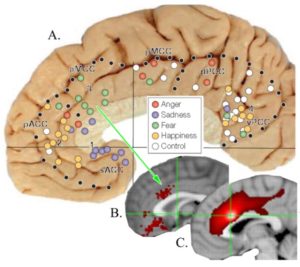 A number of observations from the top plate are of particular interest in the context of connectivity.
A number of observations from the top plate are of particular interest in the context of connectivity.
1. “Fear” activations are in ventral aMCC on the cingulate gyral surface and subsequent connection studies have amplified this finding (C.). The extensive projections into the cingulate premotor areas (C.) in the cingulate sulcus emphasize the likely role of ventral aMCC fear as a premotor signal that guides avoidance behaviors.
2. Anger was sparsely distributed in most subregions but virtually non-detectable in the Neurosynth meta analysis.
3. Sadness was elevated in sACC in the early study but there were too few studies in Neurosynth as was true for happiness to localize a cluster of peak activations.
4. Non-emotion controls (scripts and film strips without emotional content; white circles) were highest in PCC indicating a limited role in primary emotions.
From these observations the following conclusions appear reasonable. Fear is a premotor pain signal for avoidance responses given the overlap of these two functions. As the cluster of active fear voxels is on the gyral surface (B.), it must be determined how they access cingulate and dorsal premotor areas (C.). Beyond the above figure, for this we turn to cutouts of an early study by Pandya et al. (1981) as shown in the figure below. These injections of tritiated-amino acids show anterograde transport and the injection in C. is in aMCC. While it shows a wide distribution in cingulate cortex including the cingulate sulcus suggesting a cingulate integrative function, there are limited projections dorsally to supplementary and pre-supplementary motor cortices. The injection in panel B. is a larger injection mainly in the pMCC. These projections terminate in all cingulate motor cortex in the sulcus and extend into pre-supplementary cortex. These findings confirm the hypothesis that fear on the gyral surface has access to motor systems and likely participate in pain avoidance behaviors. Finally, activated voxels in sACC and autonomic projections to the brainstem suggest that skeletomotor (MCC) functions are dissociated in cingulate cortex, although temporal coordination of the two may produce a joint skeletomotor/autonomic output system as suggested in the next figure and associated text.
These injections of tritiated-amino acids show anterograde transport and the injection in C. is in aMCC. While it shows a wide distribution in cingulate cortex including the cingulate sulcus suggesting a cingulate integrative function, there are limited projections dorsally to supplementary and pre-supplementary motor cortices. The injection in panel B. is a larger injection mainly in the pMCC. These projections terminate in all cingulate motor cortex in the sulcus and extend into pre-supplementary cortex. These findings confirm the hypothesis that fear on the gyral surface has access to motor systems and likely participate in pain avoidance behaviors. Finally, activated voxels in sACC and autonomic projections to the brainstem suggest that skeletomotor (MCC) functions are dissociated in cingulate cortex, although temporal coordination of the two may produce a joint skeletomotor/autonomic output system as suggested in the next figure and associated text.
The injection in B. above is located in pMCC and shows a strikingly different pattern of connections from that of aMCC (C., D.). Not only does it have extensive projections throughout cingulate cortex, it has major projections to dorsal supplementary and pre-supplementary cortices. Two issues are raised about pMCC. First, it has essentially no role in emotion as noted above. Even non-emotion controls do not activate it; yet it has massive dorsal projections to cingulate and supra-cingulate motor areas. Frot et al. (2008) evoked responses <1 second after a noxious laser stimulus in epileptic patients with subdural electrodes. By exploring the whole rostrocaudal extent of cingulate cortex, they found that only a restricted area in the pMCC responded. The cingulate nociceptive responses showed two components, of which the earliest peaked at latencies similar to those in SII. These data provide evidence that activations underlying the processing of nociceptive information can occur simultaneously in the medial and lateral pain systems. Their final conclusion makes a pivotal point: “the existence of short-latency pMCC responses to pain indicates that the ‘medial pain system’ is not devoted exclusively to the processing of emotional information, but is also is involved in fast attentional orienting and motor withdrawal responses to pain inputs.” Thus, based on these and above studies, pMCC is not engaged mainly in emotion.
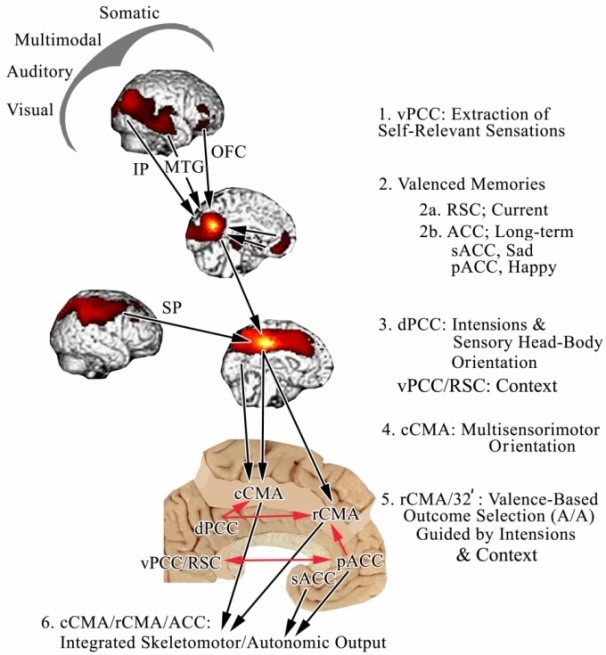
Based on circuitry and functional studies, we proposed a six-stage model of how sensory information has access to cingulate cortex to evoke coordinated skeletomotor/ autonomic responses (Vogt and Laureys, 2009) with this figure from this article. The model postulates that sensory information relating to objects and events in their context has access to the vPCC/RSC when they are personally relevant and may require movement. The medial and lateral surfaces with significant basal Correlated Clusters Of Interest (bCCOI) from seeds in PET studies are coregistered to the medial surface flat map at the bottom of the figure to expose the CPMAs and RSC (Vogt and Laureys, 2009). The first stage involves extraction of self-relevant information from all sensory cortices; this information is extracted from memory via the RSC (not shown as it is intermingled with the vPCC bCCOI in 2. and ACC as shown with the arrows from pACC and sACC. These codes are used to guide behavioral outputs such as approach and avoidance movements (5. A/A). It is proposed that PCC is involved at all stages of processing including intentional mediation of CPMA outputs (red arrows controlling CPMA output to the spinal cord, striatum and other skeletomotor centers and autonomic output from sACC; for details on the latter (Vogt and Vogt, 2009).
Valenced memories are critically dependent on reciprocal interactions between ACC and vPCC. The dPCC receives visual input and drives orientation of the head and body too such stimuli in the caudal cingulate premotor area (cCPMA; also caudal CMA) for attention and intentions along with aMCC. As noted above, OFC input to vPCC sets value states that maintain goals for action. The rCPMA (also rCMA) evaluates rewarded and punished outcomes and adjusts movement sequences based on feedback mechanisms to accomplish intended movements in particular contexts such as contextual fear learning. The coordinated output of both cingulate premotor areas and sACC then simultaneously drive relevant skeletomotor and autonomic motor systems.
With the above model, we begin to see a more refined definition of attention than the one that emerges from large strokes. Attention in this model reflects cingulate activity that addresses the needs and goals of the individual in specific contexts. It focuses on particular aspects of the environment and determines how to achieve a goal. With feedback from each movement, adjustments are made to refine approach or avoidance responses.
One must wonder why there is such an elegant cytological and connectional differentiation along the cingulate gyrus and precise functional segregations. It appears that emotion is not processed in terms of broad perceptual, introspective viewpoints as often attributed to cingulate cortex. Rather, aggregates of neurons extract sensory context information and code events that have access to cingulate cortex through vPCC and these codes are the substrate for further cingulate processing, orientation and premotor control. I proposed in 2009 (Library #28) that while emotions may be unified perceptual events, they are handled in different ways throughout cingulate cortex. Here we see an expansion of such perspectives.
ACC, primary limbic cortex for primary emotions and autonomic regulation aMCC, limbic premotor cortex for selection of action outcomes and goal-oriented behaviors pMCC, limbic premotor orientation cortex for attention driven shifts in processing & vestibular orienting RSC, personal orientation in space and navigation maps for approach/avoidance dPCC, limbic multimodal association cortex vPCC, limbic sensory assessment cortex for personal relevance and a gateway to limbic processing
Thus, limbic/emotion functions can be differentiated into subcomponents based on the 8- subregion model. Two issues in particular remain open for further research. First, the functions of anterior and intermediate RSC are not known possibly because we have not yet demonstrated the architecture of the rostral extent of these subregions. Second, the connection mechanisms and subregions involved in different aspects of social interactions are not known. While empathy is part of interpersonal interactions and is mediated by MCC (below), this is hardly a complete accounting of the role of cingulate cortex therein.
Models of brain function require validation from clinical studies including transcranial direct current stimulation of MI as shown above, localization of drug actions and damage associated with unique cingulate subregions. There are still few studies that address drug actions in specific parts of cingulate cortex but two consider the role of ibuprofen and ketamine in pain relief. Hodkinson et al. (2015) showed that the commonly used nonsteroidal anti-inflammatory drug ibuprofen does not alter cerebral blood flow under pain-free conditions, but blood flow following third molar extraction was significantly reduced in aMCC in conjunction with a significant reduction in pain ratings following ibuprofen administration. The adjacent figure shows this pattern of binding that extends beyond aMCC into pACC and less so into pMCC. In another study, Sprenger et al. (2006) evaluated healthy volunteers with fMRI while receiving noxious thermal stimuli in conjunction with placebo or increasing doses of ketamine. The ketamine isomer used is thought to have stronger analgesic potency and a preferable side effect profile including less intense psychomimetic adverse effects which seems to be consistent with our views of MCC functions. During placebo administration, the pain network was activated including aMCC. Pain unpleasantness and intensity ratings declined as ketamine dosage was increased and decreased pain perception with ketamine was dose-dependent and associated with reduction of pain-induced activations. These two studies confirm the role of aMCC in pain and suggest that more effective pain relief can be achieved with drugs that target aMCC. They also confirms the predictive power of the 8-subregion approach.
Hodkinson et al. (2015) showed that the commonly used nonsteroidal anti-inflammatory drug ibuprofen does not alter cerebral blood flow under pain-free conditions, but blood flow following third molar extraction was significantly reduced in aMCC in conjunction with a significant reduction in pain ratings following ibuprofen administration. The adjacent figure shows this pattern of binding that extends beyond aMCC into pACC and less so into pMCC. In another study, Sprenger et al. (2006) evaluated healthy volunteers with fMRI while receiving noxious thermal stimuli in conjunction with placebo or increasing doses of ketamine. The ketamine isomer used is thought to have stronger analgesic potency and a preferable side effect profile including less intense psychomimetic adverse effects which seems to be consistent with our views of MCC functions. During placebo administration, the pain network was activated including aMCC. Pain unpleasantness and intensity ratings declined as ketamine dosage was increased and decreased pain perception with ketamine was dose-dependent and associated with reduction of pain-induced activations. These two studies confirm the role of aMCC in pain and suggest that more effective pain relief can be achieved with drugs that target aMCC. They also confirms the predictive power of the 8-subregion approach.
The concept of selective vulnerability of cortical units to different diseases based on variations in neurochemistry was first recognized by C. and O. Vogt (1922; also Hopf, 1970). The original Vogts found that diseases of the nervous system are often confined to structures they termed “topistic” units. They defined “pathoclisis” as the phenomenon that only part of the organism undergoes pathological changes when the entire organism was exposed to a noxious agent and concluded that, since topistic units respond differently, each must have its own unique physiochemical properties.
As important as cytoarchitecture, circuitry, chemoarchitecture and functions are, these are just factors which together contribute to the selective vulnerabilities of each region or subregion of the cingulate cortex to particular diseases. Indeed, selective disease vulnerability provides an independent method of testing the veracity of the four-region, neurobiological model. Moreover, unique circuit chemistries and activations during particular conditions can lead to these vulnerabilities, such as during chronic pain or intense emotional stress.
To the extent that the ACC/MCC distinction has value for assessing disease etiologies, it predicts a selective vulnerability of MCC to another class of disease; i.e., those related to sensorimotor and cognitive processing rather than affect and valenced memory storage. The MCC has a prominent role in the anticipation of painful events as discussed by Porro and Lui (Library #43) and in acute pain processing. This excitatory engagement during pain and its anticipation appears to generate a selective vulnerability in MCC. Importantly, neuropathic pain and some other chronic pain syndromes produce selective reductions in activity in MCC as discussed by Kulkarni et al. (2005) and Vogt et al. (Library #37).
Albuquerque et al. (2006) reported that noxious thermal stimulation on facial skin of patients with burning mouth disorder produces a qualitatively different pattern of activation than that in control subjects. 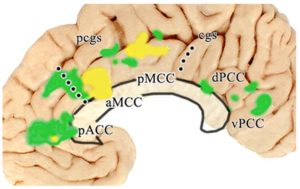 Their use of Brodmann’s anterior cingulate concept, however, distracts from the qualitative differences in activation patterns instead of quantitative (algebraic) changes at the same sites. These activations are plotted in the adjacent figure onto one of our cases with the same color code for patients (yellow) and controls (green) as in the original article. Indeed, during acute noxious stimulation of the face, the control subjects activate pACC and aMCC that are likely associated with the affective component of pain, while the patients activate only MCC. The responses may be driven more by previous/conditioned responses and enhanced anticipation of the pain than a simple pain affect as in control subjects. Activation of different regions indicates a qualitatively different response pattern in the patient population.
Their use of Brodmann’s anterior cingulate concept, however, distracts from the qualitative differences in activation patterns instead of quantitative (algebraic) changes at the same sites. These activations are plotted in the adjacent figure onto one of our cases with the same color code for patients (yellow) and controls (green) as in the original article. Indeed, during acute noxious stimulation of the face, the control subjects activate pACC and aMCC that are likely associated with the affective component of pain, while the patients activate only MCC. The responses may be driven more by previous/conditioned responses and enhanced anticipation of the pain than a simple pain affect as in control subjects. Activation of different regions indicates a qualitatively different response pattern in the patient population.
Based on the reciprocal suppression model in which ACC and MCC can inhibit each other when active, the activity in MCC might be enhanced in association with premotor processing and a cognitive effort leads to reducing activity, and presumably emotion that is normally generated in ACC. Since all responses were aggregated under a single “ACC region” in the original report, the pivotal dissociation between the control and pain groups was not fully appreciated. Furthermore, changes in PCC might also be better clarified in the regional model where it appears that burning mouth patients had no activation, while controls had activity mainly in vPCC and to a lesser extent in dPCC. It is quite likely that keys to the etiology of burning mouth disorder lie in the altered activation of qualitatively unique parts of the cingulate gyrus. The four-region neurobiological model requires a consideration of the connections and neurotransmitter systems that are modified by this chronic pain state to determine the physical substrate that is altered by chronic trigeminal activation.
Finally, while the complete mechanisms of social interactions are not understood in terms of structure/function units in cingulate cortex and their connections, empathy is certainly part of these complex functions. Empathy refers to sharing the emotions of others and is central to successful socializing. It is the ability to understand what others feel, be it an emotion or a sensory state. Accordingly, empathic experience enables us to understand what it feels like when someone else experiences sadness or happiness and also pain or touch and is associated with activation in neural structures that are also active during the first-hand experience of that emotion. For example, cutaneous pain activates mainly MCC and this is one of the parts of the brain involved in pain empathy; i.e., part of the neural activity shared between self- and other-related experiences.
Singer et al. (2004) reported that actual pain to oneself mainly activates aMCC (coded red in this figure), while observing the pain in another person (a loved one in this instance) activated mainly pMCC (coded green). There are two sites in each cingulate region where the red and green overlap but the meaning of this is still unclear; they may be associated with coordinating responses between the two regions. IThus, if we observe someone in pain, this response helps us respond to the other person’s pain in a caring manner as we would our own pain. Also, other parts of the brain are engaged in empathy, not just cingulate cortex. Empathy also helps a social group to share experiences that are helpful to survival. An example of this is a monkey troop that is threatened by an intruder and they coalesce as a group for protection in what is termed “emotional contagion.” Group fleeing behavior of humans during a mass shooting incident suggests that a similar response is at work in our species.
There are two sites in each cingulate region where the red and green overlap but the meaning of this is still unclear; they may be associated with coordinating responses between the two regions. IThus, if we observe someone in pain, this response helps us respond to the other person’s pain in a caring manner as we would our own pain. Also, other parts of the brain are engaged in empathy, not just cingulate cortex. Empathy also helps a social group to share experiences that are helpful to survival. An example of this is a monkey troop that is threatened by an intruder and they coalesce as a group for protection in what is termed “emotional contagion.” Group fleeing behavior of humans during a mass shooting incident suggests that a similar response is at work in our species.
Thus, these and many other clinical studies validate the 4 region/8 subregion models of cingulate cortex. The value of these models work in the reverse direction as well; i.e., they provide new insights into the functions, circuitries and information processing of each cingulate division. As human imaging research continues to slowly adopt these models, a greater appreciation of these issues will emerge.
Albuquerque RJC, de Leeuw R, Carlson CR, Okeson JP, Miller CS, Andersen AH (2006) Cerebral activation during thermal stimulation of patients who have burning mouth disorder: An fMRI study. Pain 122:223-234.
Bar M, Aminoff E (2003). Cortical analysis of visual context. Neuron 38:347-358.
Beckmann M, Johansen-Berg H, Rushworth MFS (2009) Connectivity-based connection parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci 29:1175–1190.
Bench CJ, Frith CD, Grasby PM, et al. (1992) Patterns of cerebral activation during the Stroop colour word interference task: a positron emission tomography study. Neuropsychologia 31:907-922.
Bentley DE, Derbyshire SWG, Youell PD, Jones AKP (2003) Caudal cingulate cortex involvement in pain processing: an inter-individual laser evoked potential source localization study using realistic head models. Pain 102:265-271.
Biber MP, Kneisley LW, LaVail JH (1978) Cortical neurons projecting to the cervical and lumbar enlargements of the spinal cord in young and adult rhesus monkeys. Exp Neurol 59:492-508.
Bogousslavsky J, Regli F. (1990) Anterior cerebral artery territory infarction in the Lausanne Stroke Registry clinical and etiologic patterns. Arch Neurol 47:144-150.
Bohlhalter S, Goldfine A, Matteson S, et al. (2006) Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain 129, 2029–2037. doi:10.1093/brain/awl050
Braak H (1976) A primitive gigantopyramidal field buried in the depth of the cingulate sulcus of the human brain. Brain Res 109:219-233.
Broca P (1878) Anatomic comparée des circonvolutions cérébrales. Le grand lobe limbique et la scissure limbique dans la série des mammiféres. Rev Anthropol 1, Ser 2:456-498.
Bruneau EG, Pluta A, Saxe R (2012) Distinct roles of the ‘Shared Pain’ and ‘Theory of Mind’ networks in processing others’ emotional suffering. Neuropsychologia 50 (2012) 219–231.
Bush G (2009) Dorsal anterior midcingulate cortex : Roles in normal cognition and disruption in attention-deficit/hyperactivity disorder. In: Vogt BA (ed.) Cingulate Neurobiology and Disease. Oxford University Press, London. pp. 245-274.
Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cog Sci 4:215-222.
Bush G, Whalen PJ, Rosen BR, et al. (1998) The counting Stroop: An interference task specialized for functional neuroimaging-Validation study with functional MRI. Hum Brain Mapp 6:270-282.
Bush G, Vogt BA, Holmes J, et al. (2002) Dorsal anterior cingulate cortex: A role in reward-based decision-making. Proc Natl Acad Sci 99:523-528.
Carter CS, Brauer TS, Barch JM, et al. (1998) Anterior cingulate cortex, error detection, and on-line monitoring of performance. Science 280:747-749.
Carter CS, MacDonald AM, Botvinick M, et al. (2000) Parsing executive processes: strategic vs evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci 97:1944-1948.
Charvet CJ, Hof PR, Raghanti MA, et al. (2017) Combining diffusion magnetic resonance tractography with stereology highlights increased cross-cortical integration in primates J Comp Neurol 525:1075–1093.
Chiu WZ, Papma JM, de Koning I, et al. (2012) Midcingulate involvement in progressive supranuclear palsy and tau positive frontotemporal dementia. J Neurol Neurosurg Psychiatry 83:910e915. doi:10.1136/jnnp-2011-302035.
Corbetta M, Miezin FM, Dobmeyer S, et al. (1991) Selective and divided attention during visual discrimination of shape, color, and speed: Functional anatomy by positron emission tomography. J Neurosci 11:2383-2402.
Damasio AR, Tranel D, Damasio H (1990) Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res 41:81-94.
Dodell-Feder D, Koster-Hale J, Bedny M, Saxe R (2011) fMRI item analysis in a theory of mind task. NeuroImage 55:705–712.
Dum RP, Strick PL (1991) The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11:667-689.
Dum RP, Strick PL (1993) Cingulate motor areas. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt, M. Gabriel, eds.), pp. 415-441. Birkhäuser, Boston.
Eddy CM, Cavanna AE, Rickards HE, Hansen PC (2016) Temporo-parietal dysfunction in Tourette syndrome: Insights from an fMRI study of Theory of Mind. J Psychiatric Res 81:102e111.
Enzi B, de Greck M, Prösch U, et al. (2009) Is our self nothing but reward? Neuronal overlap and distinction between reward and personal relevance and its relation to human personality. PLoS ONE 4(12):e8429. doi:10.1371/journal.pone.0008429
Escobedo F, Fernández-Guardiola A, Solis G (1973) Chronic stimulation of the cingulum in humans with behavior disorders. In "Surgical Approaches in Psychiatry" (L. V. Laitinen, K. E. Livingston, eds.), pp. 65-68. Lancaster (UK), MTP, Baltimore.
Fellows LK, Farah MJ (2005) Is anterior cingulate cortex necessary for cognitive control? Brain 128:788-796.
Freeman RD, Fast DK, Burd L, et al. (2000) An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol 42: 436–47.
Frot M, Mauguière F, Magnin M, Garcia-Larrea L (2008) Parallel processing of nociceptive A-δ inputs in SII and midcingulate cortex in humans. J Neurosci 28(4):944–952.
Ghaem O, Mellet E, Crivello F, et al. (1997) Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. NeuroReport 8, 739-744.
Grabenhorst F, Rolls ET (2011) Value, pleasure, and choice in the ventral prefrontal cortex. Trends in Cog Sci. 15:56-67.
Grabenhorst F, Rolls ET, Bilderbeck A (2008) How cognition modulates affective responses to taste and flavor: top down influences on the orbitofrontal and pregenual cingulate cortices. Cerebral Cortex, 18:1549-1559.
Hadland KA, Rushworth MFS, Gaffan D, Passingham RE (2003) The anterior cingulate and reward-guided selection of actions. J Neurophysiol 89:1161-1164.
Hayden BY, Pearson JM, Platt ML (2011) Neuronal basis of sequential foraging decisions in a patchy environment. Nature Neurosci, 14(7):933-939.
Hodkinson DJ, Khawaja N, O’Daly O, et al. (2015) Cerebral analgesic response to nonsteroidal anti-inflammatory drug ibuprofen. Pain 156:301-1310.
Hopf A (1970) Oskar Vogt; 100th anniversary of his birthday. J Hirnforschung 12:1-10.
Huster RJ, Eichele T, Enriquez-Geppert S, et al. (2011) Multimodal imaging of functional networks and event-related potentials in performance monitoring. NeuroImage 56:1588–1597.
James W (1884) What is emotion? Mind 9:188-205.
Kovács AM, Kühn S, Gergely G, et al. (2014) Are All beliefs equal? Implicit belief attributions recruiting core brain regions of Theory of Mind. PLoS ONE 9(9):e106558. doi:10.1371/journal.pone.0106558
Koyama T, Kato K, Tanaka YZ, Mikami A (2001) Anterior cingulate activity during pain-avoidance and reward tasks in monkeys. Neurosci Res 39:412-430.
Krienen FM, Yeo BT, Ge T et al. (2016) Transcriptional profiles of supragranular-enriched genes associate with corticocortical network architecture in the human brain. Proc Nat Acad Sci E469-E478. doi: 10.1073/pnas.1510903113.
Kulkarni B, Bentley DE, Elliott R, et al. (2005) Attention to pain localization and unpleasantness discriminate the functions of the medial and lateral pain systems. Eur J Neurosci, 21:3133-3142.
Kwan CL, Crawley AP, Mikulis DJ, Davis KD (2000) An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain 85:359-374.
Lane RD, Fink GR, Chau PM-L, Dolan RJ (1997) Neural activation during selective attention to subjective emotional responses. NeuroReport 8:3969-3972.
Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS (2005) How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci 22:495–504.
Lewin W, Whitty CW (1960) Effects of anterior cingulate stimulation in conscious human subjects. J Neurophysiol 23:447.
Lin N, Yang X, Li J, et al. (2018) Neural correlates of three cognitive processes involved in theory of mind and discourse comprehension. Cog Affect Behav Neurosci 18:273–283 doi.org/10.3758/s13415-018-0568-6
Lord AR, Li M, Demenescu LR, van den Meer J, Borchardt V, Krause AL, Heinze H-J, Breakspear M and Walter M (2017) Richness in functional connectivity depends on the neuronal integrity within the posterior cingulate cortex. Front. Neurosci. 11:184. doi: 10.3389/fnins.2017.00184
Luppino G, Matelli M, Camarda RM, et al. (1991) Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: An intracortical microstimulation study in the macaque monkey. J Comp Neurol 311:463-482.
MacLean PD (1990) The Triune Brain in Evolution: Role of Paleocerebral Functions. Plenum Press, NY.
Marino R (1976) The anterior cerebral artery: I. Anatamo-radiological study of its cortical territories. Surg Neurol 5:81-87.
Matelli M, Luppino G, Rizzolatti G (1991) Architecture of superior and mesial Area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol 311:445-462.
Mellet E, Bricogne S, Tzourio-Mazoyer N, et al. (2000) Neural correlates of topographic mental exploration: the impact of route versus survey perspective learning. NeuroImage 2:588-600.
Mesulam M-M, Nobre AC, Kim YH, Parrish TB (2001) Heterogeneity of cingulate contributions to spatial attention. NeuroImage 13:1065-1067.
Meyer G, McElhaney M, Martin W, McGraw C P (1973) Stereotactic cingulotomy with results of acute stimulation and serial psychological testing. In "Surgical Approaches in Psychiatry" (L. V. Laitinen and K. E. Livingston, Eds.), pp. 39-58. Lancaster (UK), MTP, Baltimore.
Mitchell RLC, Phillips LH (2015) The overlapping relationship between emotion perception and theory of mind. Neuropsychologia 70:1–10.
Mohr C, Binkofski F, Erdmann C, et al. (2005) The anterior cingulate cortex contains distinct areas dissociating external from self-administered painful stimulation: a parametric fMRI study. Pain 114:347-357.
Murtha S, Chertkow H, Beauregard M, et al. (1996) Anticipation causes increased blood flow to the anterior cingulate cortex. Hum Brain Mapp 4:103-112.
Nimchinsky EA, Vogt BA, Morrison JH, Hof PR (1995) Spindle neurons of the human anterior cingulate cortex. J Comp Neurol 355:27-37.
Olson CR, Musil SY, Goldberg ME (1996) Single neurons in posterior cingulate cortex of behaving macaque: Eye movement signals. J Neurophysiol 76:3285-3300.
Pandya DN, Van Hosen GW, Mesulam M-M (1981) Efferent connections of the cingulate gyrus in the rhesus monkey. Exper Brain Res 42:319-330.
Papez JW (1937) A proposed mechanism of emotion. Arch Neurol Psychitary 38:725-733.
Paradiso S, Johnson DL, Andreasen NC, et al. (1999) Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. Am J Psychiatry 156:1618-1629.
Pardo JV, Pardo PJ, Janer KW, Raichle ME (1990) The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci USA 87:256-259.
Paus T (2001) Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2:417-424.
Perlmutter D, Rhoton AL (1978) Microsurgical anatomy of the distal anterior cerebral artery. J Neurosurg 49:204-228.
Petit L, Courtney SM, Ungerleider LG, Haxby JV (1998) Sustained activity in the medial wall during working memory delays. J Neurosci 18:9429-9437.
Phan KL, Wager T, Taylor SF, Liberzon I (2002) Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage 16:331-348.
Pool JL, Ransohoff J (1949) Autonomic effects on stimulating rostral portion of cingulate gyri in man. J Neurophysiol 12:385-392.
Pool JL (1954) The visceral brain of man. J Neurosurg 11, 45-63.
Posner MI, Peterson SE, Fox PT, Raichle ME (1988) Localization of cognitive operations in the human brain. Science 240:1627-1631.
Raichle ME (2000) The neural correlates of consciousness: An analysis of cognitive skill learning. In: “The New Cognitive Neurosciences” (M.S. Gazzaniga, Ed.), pp. 1305-1318, The MIT Press, Cambridge, MA.
Richer F, Martinez M, Robert M, et al. (1993) Stimulation of human somatosensory cortex: Tactile and body displacement perceptions in medial regions. Exper Brain Res 93:173-176.
Ring A, Waddington MM (1968) Roentgenographic anatomy of the pericallosal arteries. Am J Roentgen 104:109-118.
Rolls ET (2014) Emotion and Decision-making Explained. Oxford University Press.
Rudge P, Warrington EK (1991) Selective impairment of memory and visual perception in splenial tumors. Brain 114:349-360.
Shibata H, Yukie M (2003) Differential thalamic connections of the posteroventral and dorsal posterior cingulate gyrus in the monkey. Eur J Neurosci 18:1615-1626.
Shima K, Aya K, Mushiake H, et al. (1991) Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J Neurophysiol 65:188-202.
Shima K, Tanji J (1998) Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282:1335-1338.
Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD (2004) Empathy for pain involves the affective but not sensory components of pain. Science 303:1157-1162.
Smith GE (1907) A new topographic survey of the human cerebral cortex, being an account of the distribution of the anatomically distinct cortical areas and their relationship to the cerebral sulci. J Anat 41:237-254. See Vogt et al. (2003) for medial surface image from this study.
Sprenger T, Valet M, Woltmann R, et al. (2006) Imaging pain modulation by subanesthetic S-(+)-ketamine. Anesth Analg 103:729-737.
Stefani MA, Schneider FI, Marrone ACH, et al. (2000) Anatomic variations of anterior cerebral artery cortical branches. Clin Anat 13:231–236.
Stein DJ, Phillips KA, Bolton D, et al. (2010) What is a mental/psychiatric disorder? From DSM-IV to DSM-V. Psychol Med 40(11)1759-1765.
doi: 10.1017/S003329170999261
Sugiura M, Shah NJ, Zilles K, Fink GR (2005) Cortical representations of personally familiar objects and places: Functional organization of the human posterior cingulate cortex. J Cog Neurosci 17:1-16.
Takahashi N, Kawamura M, Shiota J, et al. (1997) Pure topographic disorientation due to right retrosplenial lesion. Neurology 49:464-469.
Talairach J, Bancaud J, Geier S, et al. (1973) The cingulate gyrus and human behavior. Electroencephalogr Clin Neurophysiol 34:45-52.
Talairach J, Tournoux P (1988) "Co-Planar Stereotaxic Atlas of the Human Brain." Thieme Medical Publishers, New York.
van Heukelum S, Mars RB, Guthrie M, Buitelaar JK, Beckmann CF, Tiesinga PHE, Vogt BA, Glennon JC, Havenith MS (2020) Where is cingulate cortex? A cross-species view. Trends Neurosci., 43(5):285-299.
Vogt BA (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 6(7):533-44. PubMed PMID: 15995724; PubMed Central PMCID: PMC2659949.
Vogt BA (2014) Submodalities of emotion in the context of cingulate subregions. Cortex 59:197-202.
Vogt BA (2015) Mapping Cingulate Subregions. In: Arthur W. Toga (ed) Brain Mapping: An Encyclopedic Reference, vol. 2, pp. 325-339. Academic Press: Elsevier.
Vogt BA (2016) Midcingulate cortex: Structure, connections, homologies, functions and diseases. J Chem Neuroanat 74, 28-46, doi:10.1016/j.jchemneu.2016.01.010
Vogt BA, Sikes RW (2009) Cingulate nociceptive circuitry and roles in pain p[rocessing: The cingulate premotor pain model. In: Vogt BA (ed.) Cingulate Neurobiology and Disease. Oxford University Press, London, pp. 311-338.
Vogt BA, Vogt LJ (2009) Opioids, placebos and descending control of pain and stress systems. In: Vogt BA (ed.) Cingulate Neurobiology and Disease. Oxford University Press, London, pp. 339-364.
Vogt BA, Berger GR, Derbyshire SWG (2003) Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci 18:3134-3144.
Vogt BA, Laureys S (2009) The primate posterior cingulate gyrus: Connections, sensorimotor orientation, gateway to limbic processing. In: Vogt BA (ed.) Cingulate Neurobiology and Disease, Oxford University Press, pp.275-308.
Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR (1995) Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J Comp Neurol 359:490-506.
Vogt BA, Vogt LJ (2009) μ-Opioid receptors, placebo map, descending systems, and cingulate-mediated control of vocalization and pain. In: Vogt BA (ed.) Cingulate Neurobiology and Disease, Oxford University Press, pp. 339-364.
Vogt BA, Vogt LJ, Laureys S (2006) Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage 29:452-466.
Vogt C, Vogt O (1922) Erkrankungen der Groβhirnrinde im Lichte der Topistik, Pathoklise und Pathoarchitektonik. J Psychol Neurol (Leipzig) 28:1-171.
Waddington MM (1984) Atlas of Human Intracranial Anatomy. Academy Books: Rutland, Vermont, USA.
Wang Z, Maia TV, Marsh R, et al. (2011) The neural circuits that generate tics in Tourette’s syndrome. Am J Psychiatry 168:1326-1337.
Williams ZM, Bush G, Rauch SL, et al. (2004) Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nature Neurosci 7:1370-1375.
Yarkoni T, Poldrack R, Nichols T, et al. (2011) NeuroSynth: a new platform for large-scale automated synthesis of human functional neuroimaging data. Front. Neuroinform. Conference Abstract: 4th INCF Congress of Neuroinformatics. doi: 10.3389/conf.fninf.2011.08.00058
Zeal AA, Rhoton AL (1978) Microsurgical anatomy of the posterior cerebral artery. J Neurosurg 48:534-559.